ASBESTOS
In Chapter I (p. 2), we stressed that substantially all solids are electronegative when reduced to colloidal size and
suspended in water of low ionic concentration — in the pH range of 5 to l0.*
Thus far, we have presented a few electropositive colloids, but they achieved this state only through an applied cationic electrolyte or polyelectrolyte. But when these electropositive systems were serially and geometrically diluted, the colloid desorbed — and became ( or tended to become ) electronegative.
Chrysotile asbestos, one of the serpentine group minerals, is considered a hydrated magnesium silicate containing varying waters of crystallization. Its general formula is listed as Mg3Si2O5(OH)4 or Mg6Si4Ol0(OH)8. It is of course obvious that these expressions are identical. The former may be correctly expressed on a stoichiometric basis as 3MgO • 2SiO2 • 2H2O. The many varieties of asbestos include: anhydrous iron–magnesium silicate, sodium–iron silicate, calcium–magnesium silicate, and calcium–iron–magnesium silicate. Several well recognized types are: chrysotile, crocidolite, amosite, anthophyllite, tremolite, and actinolite. Both classification and grading are varied and complex.
It should be noted that in Nature, silicon dioxide ( as SiO2 ) is highly insoluble. However, when reacted with hot caustic soda, it forms a soluble sodium silicate**
[ ** "Soluble Silicates" — James G. Vail ( Ref. 12–10 ).]
which is a very effective anionic dispersing agent, capable of producing highly electronegative Zeta Potentials. However, if the pH of a sodium silicate solution is suitably lowered, we enter a stage of polymerization characterized by micelle formation and increased viscosity. Further additions of acid produce a rigid gel, which can harden so quickly that its removal from a container is quite difficult.
Thus, the siliceous component of asbestos per se is highly complex. When Nature couples this with magnesium, calcium and / or iron ( whose silicates are relatively insoluble ), inorganic substances of great sophistication are formed. Nature's product is much more complex than the formula would indicate.
The range of constituents ( for milled fibers ) is listed by Bergert
[ Ref. 12–32, p. 55. ] as: 35–44% SiO2; 36–44% MgO; 0–9% Fe2O3 + Al203; 0–2% CaO + Na2O; and, 12–15% H20. A typical analysis
[ "Mineral Deposits" — Lindgren — p. 395 — McGraw Hill — 1933. ]
of Canadian ( milled ) chrysotile mined about 1933, showed 42% SiO2; 42% MgO; 14% H20; 1% FeO; and, 1.7% Al203. ( Unbalanced total = 100.7%. )
A typical recent analysis of Canadian ( Thetford ) chrysotile milled fiber, given to the writer by R. C. Breiner of Nicolet Industries is: 39.05% SiO2; 3.67% Al203; 2.41% Fe2O3; 40.07% MgO; Combined Water 14.48% ( H20 ). ( Total unadjusted = 99.68% ).
Much of the confusion which exists in the literature on asbestos could have been avoided if the investigator had plainly stated whether the asbestos described was natural pure fiber ( hand–picked and hand cut ) — or a "milled" fiber. The former represents ( as closely as possible ) the product as it occurs in Nature. The latter represents the pure product — plus varying amounts of rock dust and other debris incidental to the mining, crushing, fiberizing, screening and processing of the fiber for commercial use. There is undoubtedly some variation in the pure product. But there is even greater variation in the extraneous rock dust and debris, which is always present in varying degrees and composition in the finished commercial product.
Some authorities contend that there is no
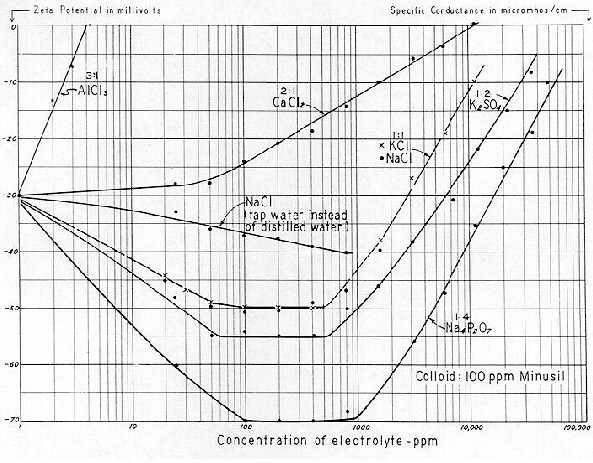
aluminum in natural asbestos, and that the reported aluminum content for the commercial product reflects only extraneous contamination inherent to normal production. Whether or not this is the case, aluminum is generally a normal constituent of the milled fiber, and it must be dealt with when the fiber is processed into the finished product. As to the stability of an asbestos slurry, the tremendous effect of the trivalent aluminum ion on Zeta Potential was demonstrated on Fig. 19. As far back as 1880, Schulze and Hardy ( correctly ) appraised its "coagulating effect" as high as even 1,000 to 5,000 times that of monovalent cations.
Most substances can readily be placed in either a mineral or organic category on the basis of appearance and other obvious characteristics. But this simple classification does not apply to asbestos. Even its mineral status relies heavily on semantics, for its highly fibrous nature seems to place it more in an organic category. The condition that one investigator will report a sample of chrysotile as being electropositive, and another finds the same sample electro–negative, would seem to throw it into a classification, which could almost be termed "hermaphroditic."
[ * Proteins are an exception, for they generally become electropositive in the pH range of 2 to 5. ]

Thus, there are many valid physicochemical reasons why asbestos is complex. Martinez and Zucker note:*
"Chrysotile and lizardite are two minerals in the serpentine group which have strikingly different physical and chemical properties. Both are hydrated magnesium silicates [ Mg6 (OH)8 Si4Ol0 ] varying only slightly in chemical composition, but having vastly different crystal structures." They ( and others ) also note that although the physicochemical properties of asbestos have been well investigated, this has produced much "irreproducible data." The writer suggests that this "irreproducibility" is due to:
2) The fact that these dissociated ions produce "bulk–stress," which has not heretofore properly been taken into account. Bulk–stress can markedly affect colloid stability.
3) The condition that "Streaming potential" has been principally employed for evaluating the physicochemical properties of asbestos. Zucker notes in the first paragraph of the Abstract of his ( Columbia University Doctorate ) thesis**
[ ** See D. W. Fuerstenau, ScD thesis, Mass. institute of Technology, 1953 — Also, G. L. Zucker, ScD thesis, Columbia University, 1959. ]
entitled A Critical Evaluation of Streaming Potential Measurements:
He closes his Introduction (p. 5) with the statement:
[ Streaming potential measurements are subject to an important source of error; an error, which may exceed the magnitude of the true streaming potential. A potential difference can arise when the cell electrodes alone undergo motion with respect to the electrolyte and is associated with the polarizability of the electrode materials. A reversible electrode in a flow system measured against an electrode in quiescent compartment has its potential changed by electrolyte flow; but two electrodes identical in geometry as well as in reversible e.m.f. exhibit no potential differences upon flow of electrolyte. ]
It is evident from the results of the present work that much of the data on streaming potentials in the literature may be inaccurate; and further, that data reported for materials in the neighborhood of their isoelectric or null potential points may be completely erroneous.
Considering the possibility that streaming potentials do not equate with Zeta Potential as determined by cell electrophoresis, it should be noted that in 1945 Irving S. Wright, M.D., of the Cornell University Medical College, presented an extensive paper on the streaming potential of blood before the Third Josiah Macy, Jr. "Conference on Blood Clotting and Allied Problems."
This paper was entitled Zeta Potential Measurement as a Tool for Studying Certain Aspects of Blood Coagulation. His apparatus and technique seem meticulous, but this research apparently did not throw significant or even mildly informative light on blood coagulation. Could this have been due to failure of streaming potential to furnish informative and / or accurate data?
The writer feels that because of inverse electro endosmosis, a condition that has not been properly recognized and taken into account, basic concepts of both streaming potential and electrocardiography may be seriously complexed — and rendered uncertain and erroneous. Since our basic concepts of adsorption in liquid–solids systems of high solids concentration seem veritable, the question arises: How does one produce, with streaming potential, the three highly pertinent curves of
Fig. 25 for the same reagent and the same colloid? (See pp. 32 to 39.) This matter will be briefly considered later herein, and in detail in Vol. II of this work.
If we seem to belabor the triple subjects of asbestos, blood coagulation, and streaming potentiality is because the writer believes that the physicochemical behavior of both asbestos and blood ( though entirely dissimilar ) presently represent the acme of ambiguity and sophistication in mineral and organic systems. To understand either, we must build our concepts step by step — from the simple to the complex. No data that stems from false assumptions or techniques can serve a useful purpose. Indications are that much data obtained from streaming potentials in the past falls into this category.
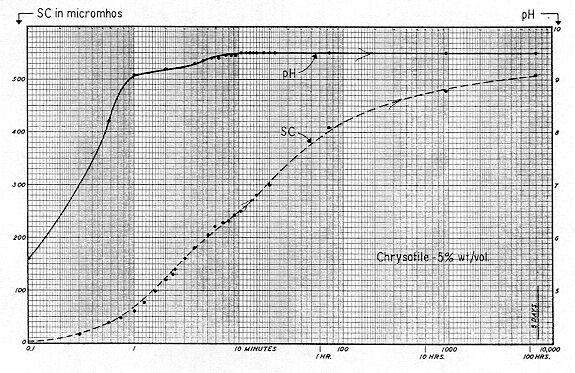
Fig. 77 shows "multiple–dilution–desorption," pH, and SC curves for milled chrysotile asbestos ( Class 7 short fibers — supplied by R. C. Breiner of Nicolet ). The sample was prepared by adding 10 grams of chrysotile to a liter of distilled water stirring for 30 minutes at 300 rev. / min. The fibers were then allowed to settle, and a 50 ml sample of the supernatant, containing ample colloids and short fibers for tracking, was removed for cell electrophoresis.
[ Paper by Wood, Horan, Sheppard & Wright. Proceedings published in 1950 by the Josiah Macy, Jr. Foundation, 565 Park Ave., N. Y. 21, N. Y. ]
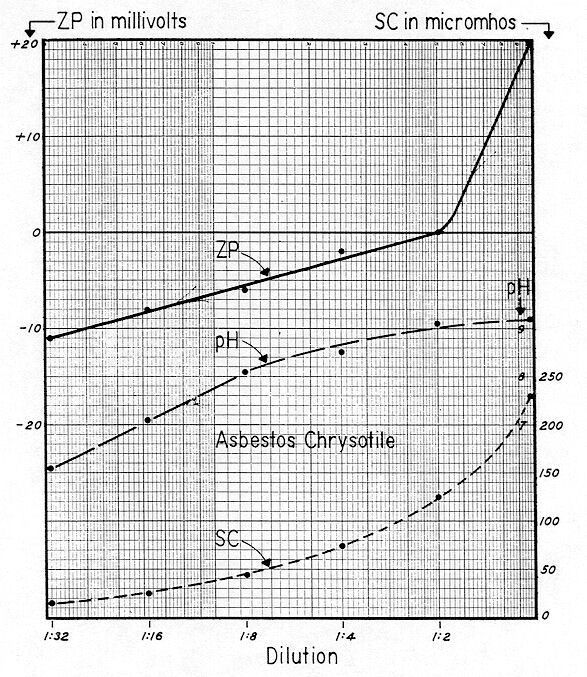
The initial ZP was +20 mv, and a 1:2 dilution lowered this to zero mv. Further dilution resulted in the colloid becoming increasingly electronegative, with a value of –11 mv at a dilution of 1:32. The curve consisted of two tangents with a short transitional segment, and the shape strongly resembled that of the cutting oil emulsion shown on Fig. 49. One must approach Zeta Potential by gradually working from simple to complex systems. Thus, many seeming inconsistencies can be obviated. In Fig. 49, we were dealing with a cationic polyelectrolyte which had been intentionally applied. In Fig. 77 it would seem that we deal with a system containing a low concentration of a 3:1 electrolyte — or a cationic polyelectrolyte — which dissolved from either the chrysotile per se or its accompanying ore dust. The normal composition of milled fibers would suggest that aluminum was the cation.
Note that the natural bulk–stress initially was 230 micromhos — and it decreased uniformly, as it did in most of the preceding curves. But there is one notable difference: In the case of Minusil ( Fig. 23, ), the bulk–stress of 830 micromhos was due to extraneous ions present on the silica, and not to solution of the silica per se. If we had thoroughly washed the Minusil in distilled water several times, dried it, and then made it up again in distilled water, we would probably have dissipated all of our bulk–stress. But if we had then soaked the Minusil in a solution of say calcium chloride — air dried it — and made it up again in suspension — our bulk–stress would have re–appeared. We emphasize that this type of "natural bulk–stress" is due to extraneous ions probably loosely held to the surface of the colloid. If these extraneous ions are readily soluble, as in the above example, the Specific Conductance of a slurry carrying these ions will rise immediately. However, if the extraneous ions are relatively insoluble, or slowly soluble ( such as calcium or magnesium carbonate ), then the Specific Conductance of such a slurry will rise slowly.

A commercial zinc, titanium, or lead oxide can have two types of bulk–stress: one associated with its manufacturing processes and occurring as described above; the other arising from slight ( but definite ) solution of the oxide per se. The first may be considered "extraneous" or "extrinsic" — and the second "innate" or "intrinsic." Upon suspending a dry colloid in distilled water, the "extraneous" ions may go into solution rapidly or slowly — depending upon the nature and rate of solubility of the compounds producing the ions, as previously noted. But the "innate" or "intrinsic" ions "peel off" much more gradually, arriving at a state of equilibrium in a matter of hours–to days–to weeks.
These concepts of solubility, and extrinsic and intrinsic ions — make for a better understanding of asbestos — or any mineral or organic solid which slowly releases ions to the bulk phase. Temporarily, we omit discussion of the pH curve of
Fig. 77, and present instead
Fig. 78, so that we may illustrate the highly dynamic properties of "milled" chrysotile.*
[ * Pure chrysotile is not herein considered because the writer wishes to demonstrate basic principles and not discuss either pure chrysotile or milled fibers. An excellent review of chrysotile ( with 49 recent references ) will be found in the November 1966 issue ( pp. 1305–1311 ) of Canadian Mining & Metallurgical Bulletin, Vol. LXIX, by Edward Martinez of the Central Research Laboratories, American Smelting and Refining Company, South Plainfield, N. J. ]
These pH and SC curves were prepared as follows: 950 ml of distilled water as added to a liter beaker, and continuously stirred at 300 rpm. The Zeta–Meter Automatic Sample Transfer was then connected to the beaker, which permitted transfer of liquid ( at room temperature ) from the beaker to the electrophoresis cell in only 15 seconds. To exclude coarse fibers, a fine filter was employed on the suction line. In this instance, the cell was employed to measure Specific Conductance only, as time did not permit the simultaneous determination of Zeta Potential.

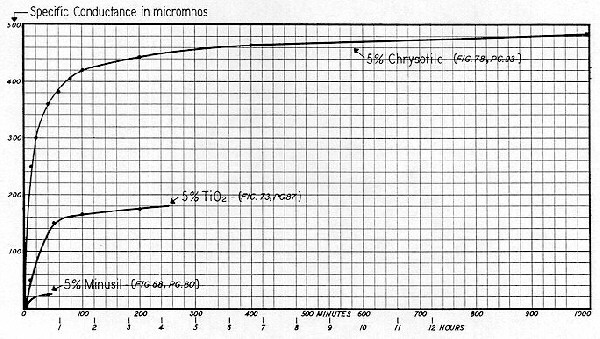
The initial Specific Conductance of the distilled water was 2 micromhos; and pH, 5.6. A fifty gram lot of chrysotile ( the same as before ) was quickly transferred to the beaker, forming a 5% suspension. Note that SC increased at a relatively rapid and constant rate during the time interval from 1 to 100 minutes, but that ions were still being liberated to the bulk after a period of even five days. As previously noted, the leveling off in the rise of Specific Conductance can best be evaluated by an arithmetic graph. The SC curve of Fig. 78 is therefore replotted on this basis and is shown on Fig. 79, together with the SC — time curves for the 0.5% slurries of Minusil ( Fig. 68 ), and titanium dioxide ( Fig. 72 ). The sizeable differences in the time required for "reasonable stabilization" are readily apparent. Natural bulk–stress, as computed by the formula
NBS = SC ÷ 2,000 X [ % (by weight) of liquid in system ] ÷ [ % (by weight) of suspended solids in system ]
was:
| System | Approximate Time Required for "Substantial Stabilization" | Terminal NBS - mg/g ( equiv. NaCl ) |
|---|---|---|
| 5% Minusil | 20 minutes | 0.2 |
| 5% TiO2 | 1 to 2 hours | 1.7 |
| 5% Chrysotile | 6 to 12 hours | 4.6 |
The practical conclusions to be drawn from these data are that each colloid system has its individual dynamic characteristics — and they should first be roughly evaluated to serve as guidelines for appropriate laboratory technique. The rise in natural bulk–stress may be slight, as with Minusil; or significant, as with this titanium dioxide; or pronounced and attenuated, as with chrysotile. Bear in mind that although bulk–stress is indicative of conditions that could significantly affect colloid stability, high bulk–stress per se is not synonymous with significant changes in ZP or stability — because this largely depends upon thetype of electrolyte being "peeled off" and released to the bulk. The curves shown on Fig. 79 simply indicate that to assure Zeta Potential values which are capable of "reasonable" repetition, we should determine the ZP of the Minusil say 30 minutes after slurrying. The ZP of this particular titanium dioxide should be run 3 to 2 hours after slurrying; and the ZP of chrysotile should preferably be determined the day following the preparation of the slurry.

We emphasize that one must exercise judgment in evaluating the possible ill effects of bulk–stress due to ageing, which may range from negligible to serious. For example, an increase or decrease of 20 mv in the ZP of a slurry ( due to ageing ) can make a marked change in the physical characteristics of the system when the initial or final ZP of the slurry ranges from zero to +25 or zero to –25 mv. This is because before ageing, the slurry could be disperse — and after ageing, coagulated — or vice versa. But if a freshly prepared slurry was dispersed to a ZP of say –60 mv by the application of an anionic surfactant, then a change of –20 mv (due to ageing) should cause no difficulty. This is because stability is generally assured in the entire electronegative range of –40 to –80 mv.
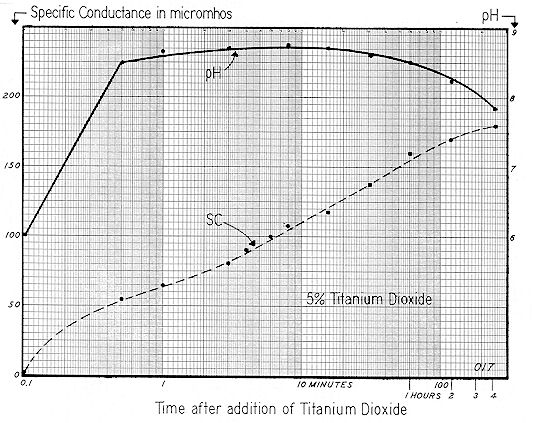
Returning to the curves of Fig. 78, Hydrogen ion concentration rose from 5.6 to 9.0 in one minute; and 9.5 in ten minutes. Undoubtedly this reflected the solution of a soluble fraction of the magnesium hydroxide component of the chrysotile. But it is also apparent that during the time interval from ten minutes to five days, pH declined from 9.5 to 9.4, while Specific Conductance continued to rise ( from 250 ) to 510 micromhos.
To further investigate this system, the agitator was shut down ( it had been in continuous operation for five days ), and the slurry ( then at SC 510 and pH 9.4 ) was permitted to settle for an hour. A sample of the clarified supernatant was then collected and vacuum filtered — twice through a 0.45 micron Millipore filter, and once through a 0.22 micron Millipore. This produced a filtrate with the clarity of distilled water and we think it substantially removed colloids 1,000 Angstroms and larger. Spectrographic analyses of the filtrate were made by Dr. Bell, Director of Lucius Pitkin, Inc.* [ * 50 Hudson Street, New York, N. Y. ] who was requested to exercise meticulous care with this sample. Results are shown in Table No. 10. Column 2 gives the probable concentration of the cations to within tolerances normal for spectrographic analysis. Total solids of the sample upon drying, burning and weighing amounted to 720 ppm. Col. 4 ( based on the weight of the oxide relative to the cation ) gives the probable ppm when weighed; and Col. 5 adjusts the first value ( since it is decidedly major ) on the basis of 720 ppm actual weight ( 900 – 720 = 180; 830 – 180 = 650 ).
From
Fig. 78 it appears that the water soluble component of the chrysotile began to dissolve immediately — releasing magnesium and hydroxyl ions to the suspending liquid. In one minute this raised pH to 9.0, and SC to 65 micromhos. Reference to
Fig. 59 or
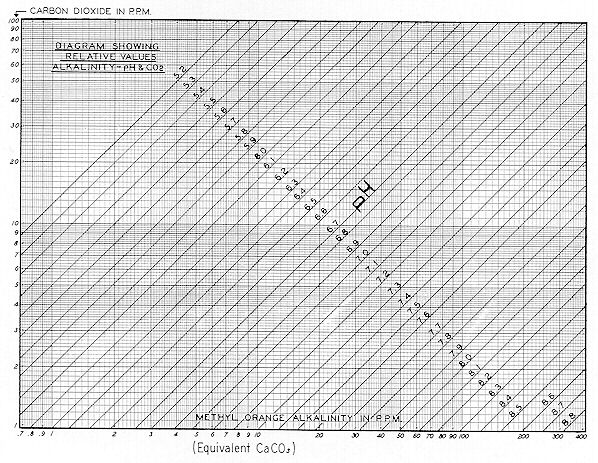
Fig. 60 shows that either 12 ppm of NaOH or 30 ppm of Na2CO3 would produce a pH of 9.6. So it would seem that the inability of the pH curve of
Fig. 78 to rise above 9.5 was due to the Mg(OH)2 being continuously converted ( by absorption of atmospheric C02 before or during stirring** )
[ ** It is also possible that this conversion from hydroxide to bicarbonate took place in situ — long before the chrysotile was mined. ]
to MgCO3 — to Mg(HCO3)2. This view seems substantiated by the condition that on Fig. 59, a dosage of 650–750 ppm of NaHCO3 produced a pH approximating 9.0 — which also agrees with the curves of Fig. 58. These generalized curves for carbonate, bicarbonate, and hydroxide alkalinity obviously rationalize dynamic systems in which cations other than sodium are involved — and there seems no need for precise stoichiometric conversion.
|
Element (1) |
Probable concentration of cation - ppm (2) |
Probable form when weighed for total solids (3) |
Probable ppm - when weighed (4) |
Adjusted ppm - when weighed (5) |
|---|---|---|---|---|
| Magnesium | 500 | MgO | 830 | 650 |
| Calcium | 25 | CaO | 36 | 36 |
| Aluminum | 5 | Al203 | 9 | 9 |
| Silicon | 5 | SiO2 | 10 | 10 |
| Iron | 5 | Fe2O3 | 7 | 7 |
| Sodium | 2.5 | Na2O | 3 | 3 |
| Potassium | 2.5 | K20 | 3 | 3 |
| Titanium | 0.5 | TiO2 | 1 | 1 |
| Copper | 0.5 | CuO | 1 | 1 |
| Total | 546 |
| 900 | 720 |
Total Solids on Evaporation — 720 ppm
Elements checked but not found:
Zinc, Manganese, Vanadium, Tin, Nickel, Chromium, Cobalt, Molybdenum, Germanium, Lithium,
Cadmium, Indium, Bismuth, Antimony, Arsenic, Lead, Thallium, Gallium, Tungsten, Zirconium, Silver, Barium, Strontium.
—
Periodic Table of Elements —
Again note from
Table 10 that magnesium, a divalent ion, amounted to 90% of the identifiable cations. From
Fig. 19 is evident that a 2:1 electrolyte with a divalent cation such as CaCl2, required a dosage — as high as 10,000 ppm to drive a dilute Minusil system to zero ZP. Therefore it seems impossible that 600 to 800 ppm of magnesium bicarbonate could produce the electropositive value of +20 mv found on
Fig. 77. Nor could the 36 ppm of divalent calcium (
Table 10 ) materially aid in lowering Zeta Potential. However, the 9 ppm of aluminum is significant because of its trivalence.
This concentration is equivalent to ( 9 X 666 ÷ 54 ) = 110 ppm of Al2(SO4)3•
18H20; or ( 9 X 133 ÷ 27 ) = 45 ppm
of aluminum chloride. This dosage of aluminum sulphate would be two to three times that employed for coagulating normal raw water colloids; and an aluminum chloride dosage of 45 ppm is eleven times that which was required to bring the Minusil system of
Fig. 19 from –30 mv to zero. Moreover, the 7 ppm of iron (
Table 10 ) represents another ( more often trivalent ) cation that is capable of lowering Zeta Potential to zero. Under favorable ( even to moderately adverse ) conditions of adsorption, it is reasonable to assume that the aluminum and iron in the chrysotile suspensions of
Fig. 77 or
Fig. 78 were fully capable of driving these systems into the electropositive range.
And so we conclude that there is nothing unusual or abnormal about the Zeta Potential curves for the cutting oil (
Fig. 49 ), or the chrysotile (
Fig. 77 ). The shape of these curves and the values of their plotted points are readily reconciled. But this calls for a reasonable working knowledge of:
Actually, there is nothing complex in the systems or the techniques thus far discussed. In fact, they lend credence to Planck's contention that the more general a natural law is, the simpler is its form.
Despite the present technical consensus that chrysotile per se is electropositive, the writer believes reported electropositivity may be due to the accompanying ( and generally extraneous ) aluminum ( and / or
ferric ) ions. This matter may soon be resolved by a series of experiments, which are being undertaken jointly with Edward Martinez, an authority on many aspects of chrysotile.
BLOOD
However, the writer believes it appropriate to now present
Fig. 80 which deals with blood, because of the strong similarity of its curves to the cutting oil curves on
Fig. 49, and the chrysotile curves on
Fig. 77 ). Having much in common, they form a basic pattern.
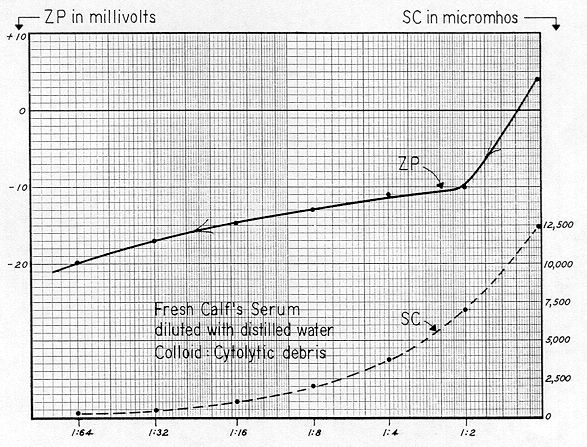
Figure 80 represents freshly drawn calf's blood — collected by the writer in centrifuge tubes and rushed to the laboratory within 40 minutes after exsanguination. Four 35 ml portions of this clotted whole blood were separately centrifuged at 3,000 G for ten minutes, producing a moderately clear serum. These portions were then combined, and a small amount of animal's clotted red cells was added. Upon stirring, these cells separated into discrete particles, and their concentration was just sufficient to enable initial tracking in the electrophoresis cell. Specific Conductance of the serum was 12,500 micromhos, which is close to the average value listed by Albritton** for human blood.
The initial Zeta Potential of this system at no dilution was +4 mv, and we stress that this represents the red cells suspended in their own bulk liquor. A 1:2 dilution with distilled water lowered Zeta Potential from +4 to –10 mv. The change in tonicity lysed the blood cells and furnished ample cytolytic debris for tracking. At the final dilution ( 1:64 ), ZP was –20 mv.
Obviously, the multiple–dilution desorption curve of this blood has shape characteristics almost identical to the MDD curve for chrysotile shown on Fig. 77. And too, since the several dilutions resulted in the colloid becoming increasingly electro–negative, the original electropositive state must have been due to the presence of a limited amount of either an adsorbed electrolyte with a trivalent cation ( such as aluminum ), or to an effective cationic polyelectrolyte.
The concentration of trivalent cations reported as being inherent to blood systems is, in the writer's view, not nearly sufficient to bring Zeta Potential to +4 mv — even to zero*
But the writer feels that prothrombin and thrombin respectively behave like ( and therefore should be considered ) extremely effective and highly specific cationic polyelectrolytes. They are inherent to mammalian blood systems, and have been meticulously investigated by Walter Seegers**
over a long period of time.
We now present a speculative hypothesis to account for the multiple–dilution desorption curve of blood shown on
Fig. 80. But the writer is convinced that any hypothesis to account for this curve, must also account for the stability of generalized blood systems. Furthermore, the basic factors, which control the stability of bovine blood, must also control the stability of human blood. While any hypothesis concerning blood stability is presently speculative, ( at the time of this writing ) our concept is based on the physicochemical principles and sequences, which we have developed thus far. All these principles are rational, optically demonstrable, and repeatable — and they apply to every colloid with which we have worked.
We postulate the sequences of blood coagulation in vitro as:
2) In health, the formed elements discrete. They do not adhere to each other — or blood vessel walls. This is due to adsorption of electronegative plasma proteins on all the formed elements, and on the vessel walls. This creates mutual repulsion.
3) It seems possible–to–probable that albumin is the principal ( or controlling ) adsorbed plasma protein which furnishes this protection; and that the mutually repelling electronegative Zeta Potential of albumin is, in turn, controlled ( principally ) by adsorbed inorganic electrolytes. Thus, we have a condition of multiple adsorption; with electrolyte A controlling anionic protein B, and protein B controlling the stability of suspensoid C.
4) When blood is "shed," prothrombin ( a substance which the writer considers a highly effective and extremely selective cationic polyelectrolyte ) is released into the system. Both the source of the prothrombin — and the method of release — have long remained unknown.
5) According to Seegers,* prothrombin is transformed into thrombin by autocatalysis and / or the action of various pro–coagulants present in the blood. Macfarlane** estimates that normal mg/l of thrombin.
6) The 150 mg / l of thrombin then combines with the 2000 to 3000 mg / l of fibrinogen, polymerizing to form fibrin — a rigid gel or clot. Fibrinogen ( in this broad frame of reference ) may be considered an anionic polyelectrolyte, and its concentration is adequate for the formation of a rigid gel.
[ The "green elements" are known to be needed by our bio–life–cycle. Today's test instruments can see these elements. — Tommy — ]
a.) bulk–stress;
b.) the effects of type and concentration of electrolytes on colloid stability;
c.) the relationship between carbonates, oxides, bicarbonates, hydroxides, carbon dioxide and pH;
d.) the dynamics of colloid systems; and
e.) a basic concept of multiple–dilution desorption curves.
Thus far, we have been sequentially developing the basic principles of Zeta Potential — from the simple to the more advanced. We now briefly depart from this pattern to discuss certain aspects of blood stability. Actually, blood systems cannot be adequately discussed in the absence of certain other germane facets of colloid stability which will be developed in Vol. II of this book.*
[ * An outline of Vol. II appears herein. ]
[ ** See Ref. 11-10, p. 7. Albritton lists the Specific Conductance of human serum ( at 25° C ) as 12,000 micromhos average; range, 11,700 to 12,400 micromhos. Later, the writer will present evidence that under our "advanced ecology of western civilization" due to processed foodstuffs — the present concentration of blood electrolytes in the human system is abnormally high. In fact, the concentration is greater than the kidneys can effectively deal with. From the standpoint of Zeta Potential, this situation appears to be a major factor in cardiovascular diseases. Additionally, highly coagulating cations are being inadvertently introduced into our foodstuff at various stages of its commercial processing, packaging, and home preparation. Much physicochemical evidence points to the above conditions as being among the principal basic causes of intravascular coagulation. ]
[ * See Ref. 11–10 in the Table of
"Blood Minor Minerals," Albritton lists the average concentration of
aluminum as follows: Blood, 0.15 mg/liter; RBC, 0.07 mg/l; Plasma, 0.46 mg/l. ]
[ ** Chairman of Department of Physiology & Pharmacology, Wayne State University School of Medicine, Detroit, Mich. ]
1) The blood of healthy humans in situ, consists essentially of formed elements ( suspensoids ), and plasma proteins ( colloids ), in aqueous suspension. Dissolved in this system are approximately 9 grams per liter of mineral salts, of which sodium chloride is the principal constituent. ( See
Tables No.
13,
14 and
15 )
[ * See Seegers, Ref. 11-6, Vol. 2. ]
[ ** See Ref. 11-1, Chapter 14 (p. 150). ]
Pertinent data regarding coagulation of human blood is summarized in Table No. 11.* [ * From Refs. 11-1, 11-3,11-6 and 6-1. ]
The albumin molecule has a length of 150 Angstroms and a width of 38 Angstroms, as compared to a length and width for fibrinogen of about 700 and 38 Angstroms respectively. The half–life of albumin approximates 14 days, and fibrinogen 4 days.
The writer is fully aware that when blood is shed," the formation of a rigid gel in vitro will be significantly attenuated if the walls of the receiving vessel are coated with silicone, paraffin, Teflon and / or like hydrophobic substances. Without doubt, this attenuation must be considered in some respect and degree a "contact factor." But the writer cannot believe that the physicochemical alteration of the system induced by this "contact" per se — is remotely capable of either triggering or converting this ( normally ) fluid system into a rigid gel. The writer's research indicates that the stability of substantially all colloid systems is dependent upon adsorption. When the surface area of a patently stable system is materially increased ( by adding a sizeable amount of "fine" colloids ), the stability of the system can be markedly altered — even completely lost. That is to say, the increased surface area will reduce the residual surfactant in the bulk — which then reduces bulk–stress — and then cause significant desorption of the original colloids of the system.**
[ ** It must be remembered that adsorption is a highly delicate, dynamic equilibrium — always requiring an appropriate residual bulk–stress for any given degree of adsorption or stability. If the surface area of the system is materially increased, the ions or polymers for this adsorption naturally come from those residing in the bulk. This tends to cause a deficiency in the bulk, which is compensated for by an actual desorption of the adsorbed colloids in the system. If the original system was either at or below peak dispersion, the introduction into the system of colloids, which materially increase surface area, must lower Zeta Potential and lower the stability of the system. ]
Thus Zeta Potential can be materially lowered, and the system brought to a state of either coagulation or gelation depending upon the nature of the stabilizing additives. This inverse physicochemical sequence is clearly inferred from the curves on Fig. 19 and others. But there is no significant increase in surface area when "shed blood" is collected in a test tube or beaker.
In the light of this, the writer believes that we must turn our attention to other areas for the answer to the triggering and physicochemical mechanism of blood which so inevitably and dramatically converts a liquid to a semi–solid state. The writer will later present herein several facets which he believes hold promise of throwing some light on this highly baffling, long–unsolved enigma.
It is well established in Physical Chemistry that a cationic and an anionic polyelectrolyte will "titrate" in a manner comparable to say acids and alkalies. However, the end point of stability is indicated by Zeta Potential — and is generally manifest through agglomeration and / or gelation, instead of change in pH. The writer believes that the conversion of prothrombin to thrombin begins immediately after blood is "shed"; and the newly–formed thrombin starts to react with the fibrinogen at once. We stress that mammalian blood represents the most highly dynamic system known; and blood coagulation ( which could more properly be termed rigid gel formation ) is the most dynamic physicochemical sequence known.
In the case of chrysotile ( Fig. 77 ), the system was electronegative immediately after adding the asbestos to the distilled water. It only gradually became electropositive as more and more aluminum ions "peeled off" and went into solution. Now with blood, there is every indication that the 2,000 to 3,000 mg / l of fibrinogen begins to react immediately with the 150 mg / l of thrombin — and within three to five minutes, a "fluid gel" is formed. This reaction continues unabated, and after another period of about five minutes, the fluid gel has developed a sufficiently interlocking and enmeshing network of micelles to bring the mass to a state which the writer terms a rigid gel. Thus it is possible to rationalize blood coagulation along lines of straight forward, thorough going Zeta Potential and Physical Chemistry. Indeed these are the same laws and principles, which we have demonstrated as governing both inorganic and organic systems, simple or complex.
|
Fraction (1) |
Approx. Molecular Weight (2) |
Isoelectric Point (3) |
Approx. conc. in Human Blood – mg / l of Plasma (4) |
|---|---|---|---|
| Albumin | 69,000 | 4.9 | 32,000 to 52,000 |
| Fibrinogen | 340,000 to 400,000 | 5.3 to 5.5 | 2,000 to 3,000 |
| Prothrombin | 62,000 to 69,000 | 4.2 | — |
| Thrombin | 34,000 to 40,000 | 3.6 | 150 |
| Total Plasma Proteins |
|
| 74,800 |
| Globulins |
|
| 20,000 |
The writer has intentionally avoided all speculative hypotheses concerning the "source" of the prothrombin and "how" it is triggered.*
[ * The unsolved mystery of what triggers blood coagulation and where the reagent comes from has long baffled scientists. And centuries of intensive research have apparently failed to throw conclusive light on this subject. Macfarlane ( Ref. 11-1, Vol. 2, p. 153 ) says: "When the earlier stages of coagulation come to be considered . . . one enters a field which is still increasing in confusion. There are no biochemical landmarks, but many inferences drawn from physiological, pathological, and clinical observations; there is no definite knowledge of any of the many factors which are supposed to be involved nor of the nature of the reaction which they are supposed to undergo." ]
But these aspects will be further considered after other pertinent facets have been developed.
The writer has long believed that through proper and meticulous employment of Zeta Potential, this puzzling enigma can be solved. In fact, in the fall of 1965, a formal Proposal was made by the writer to the National Research Council and the Department of Health, Education and Welfare, which trickled down through "channels" to the NIH and NHI. This Proposal was: "To draw blood from a normal human which, without use of any chemicals — in vivo or in vitro — would not ( naturally ) coagulate." Despite the fact that this has never been successfully accomplished, the writer and his Firm agree to bear the entire cost of this research — except that blood for the program was to be furnished by the Red Cross Blood Bank in New York City at no cost, and as required. However, an appropriate fee for this research was to be established in advance but payable only upon successful completion of this experiment.
In the ensuing correspondence, involving personnel up to and including the Director of the NIH, the writer could elicit no reaction which could be interpreted as any real interest on the part of Federal Health Authorities.**
[ ** This Proposal is still open to any person or organization of any country — whose interest is sufficient to meet its simple, straight–forward requirements. Manifestly, the successful completion of this experiment would constitute a major scientific break–through in the elucidation of blood stability and cardiovascular disease. And it could revolutionize human blood transfusion, and vastly improve our blood testing and diagnostic procedures. ]
In a letter dated May 18, 1966 to Dr. Joseph F. Hayes, Jr. of the NHI ( who had been designated by the NIH to handle this situation ), the writer inquired "If matters of this character are of no interest, what in the name of God can be the purpose of your organizations?" The writer still awaits a reply.
We stress that the physicochemical sequences outlined in the foregoing six steps relate only to the clotting of shed blood. Macfarlane has pointed out for decades that it is quite possible there is little or no relationship between coagulation in vitro — and the physicochemical characteristics of blood systems as they exist in vivo. Indeed, it would seem that clotting is mainly a homeostatic mechanism for the preservation of the species.
[ Much botanical life is also equipped with this protective mechanism. Cherry trees and acacias are good examples. ]
Frankly, the writer feels that in a relative sense entirely too much effort has been expended on the coagulation of blood in vitro — and too little on the basic cause, prevention and relief of coagulation in vivo. A brief concept of the physicochemical sequence of intravascular coagulation will be presented later herein.
We do not mean to imply that all bovine blood, or all blood from humans will compare closely with the MDD curve for calf's blood shown on Fig. 80. This pattern may vary considerably. The writer believes that in each individual blood system there is a definite quantity of fibrinogen — which in turn will react with a definite quantity of a given thrombin — to form a rigid gel.
When blood is "shed" and begins to clot, one cannot know at the outset whether:
(2.) the thrombin will become fully reacted, leaving an excess of the anionic polyelectrolyte fibrinogen.
It would seem from Fig 80 that the effective concentration of thrombin exceeded the "stoichiometric equivalent" of fibrinogen. Therefore, the serum was electropositive, as in (1). Actually, Fig. 80 represents only one of ten samples of calf's blood collected on that date. As previously noted, at "no dilution," this sample had a ZP of +4 mv. However, the other nine samples had a ZP range at "no dilution" of +5 to –6. It is doubtful, therefore, that any one sample can be considered "more normal" than another.
The writer firmly believes that the stability of blood — its dispersion and its rigid gelation — must rigorously and without deviation follow all the precepts which govern not only the colloids we have thus far considered, but also those which we will consider later. To reiterate, Zeta Potential, like the law of gravity, is a natural law. It is the basic factor controlling the great majority of aqueous systems, which include blood. The fact that blood is presently the most complex, in no way exempts it from the rules governing colloid stability — or places it in a special category. The writer is convinced that a vigorous approach through properly employed Zeta Potential would soon take much of the sophistication out of blood work.
The dozen or more established "factors" of blood coagulation continue to serve a useful purpose in that they furnish guide lines of a sort. But they should be considered only what they are: a series of rather loosely connected statements that under certain specific test conditions "normal" blood will behave in a certain manner. There is no physicochemical rationale in the "factors" upon which to systematically build a working hypothesis of blood coagulation; nor for that matter, the coagulation of any system of colloids or suspensoids — regardless of how simple. Furthermore, it is factual that no one has ever worked with a specimen of natural blood. Nor will they, until someone learns to mechanically arrest the triggering mechanism, which releases prothrombin and / or thrombin to the system. When dealing with citrated, oxalated or heparinized blood or its equivalent — we are dealing with a dynamic system after the fact; the fact being that its otherwise natural coagulation has been abruptly interrupted by chemical additives.
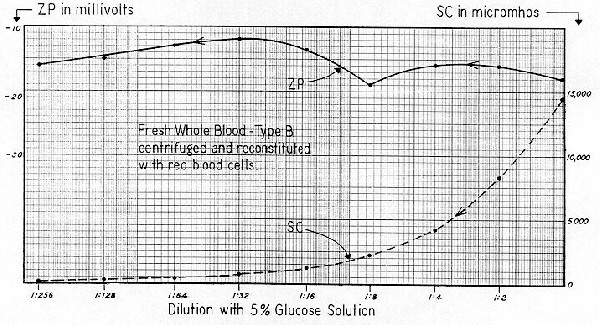
Figure 81 is a second curve for blood. This is a sample of Red Cross "ACD Whole Blood," type AB. Zeta Potential determinations were made within 24 hours after collection. The whole blood first was centrifuged at 3,000 G for 10 minutes to separate the cells from the plasma. For the purpose of tracking in the electrophoresis cell the clear liquid was reconstituted by adding a relatively high concentration of the original whole blood. This permitted high dilution without unduly depleting the RBC. Initial ZP was –18 mv.
After diluting 1:2 with 5% glucose ( which reduced SC by half without materially altering isotonicity ) ZP lowered to –16 mv. Thereafter the curve underwent changes, which were quite similar to the Purecal "D" Fig. 30. One must conclude that there is nothing abnormal or unusual about Fig. 81. Instead, it is actually obeying the same laws of bulk–stress which governed the behavior of the calcium carbonate.
Abramson established the Zeta Potential of "normal" red blood cells as –17 mv. However, one should not confuse his value with the Zeta Potential values of Fig. 81 — which range from –12 to –19 mv. Abramson suspended small concentrations of RBC in a phosphate buffer. His suspending media was therefore quite foreign to the "bulk liquor" of his original blood sample. In Fig. 81, the writer suspended the red cells in their own plasma, which, in some respects, more nearly simulates the original blood system. However, at best, both techniques are faulty. The writer's system was abnormal due to the presence of "acid–citrate–dextrose" which was initially added to the system to inhibit coagulation.
Macfarlane long has recognized the impossibility of working with blood as it exists in situ, and he has stressed that inferences drawn from blood whose coagulation was arrested, might be highly misleading. He states:*
[ * Ref. 11-1, p. 175. ]
Hageman factor deficiency produces a most curious condition characterized by a prolongation of the clotting time of the blood in glass tubes, but no definite hemorrhagic diathesis. The fact that patients with an apparent deficiency of this factor do not bleed abnormally raises rather fundamental doubts on the place of clotting in hemostasis, or alternatively, on the relevance of experiments made in glass tubes to the physiological mechanism. It might be that the blood of these patients, though failing to react to glass, reacts normally to physiological trauma. If this is so, then a large part of what has been written, thought, and taught about blood coagulation is based on one of the most extensively studied artifacts in the history of biology.
To close this section, we show eight photomicrographs ( Fig. 82 ) which are good examples of improper comprehension of dynamic systems — improper concept of Zeta Potential and improper preparation of whole blood for transfusion. They depict standard, commercial, "ACD whole blood" Type O, five days after collection from a donor at the New York Red Cross Blood Bank. Remember that this represents less than one week of storage, compared with an allowable twenty–one days.
Photographic technique was the same that was outlined in Chapters 2 and 3 (pp. 4-10), employing a Zeta–Meter microscope and Romicron camera, fitted with Polaroid back. Polaroid–Land fifteen–second film was used. A picture of this assembly is shown as Fig. 83.
A single drop of ( undiluted ) blood was placed on the glass cell holder, then covered with a standard glass slide, as in Fig. 2b. The drop was then illuminated from above ( rather than below ) and photographed at shutter speeds "as required." The agglomerates stand out as blue–tinted gel formations, in bold contrast to the "blood red" surroundings.
The agglomerates shown on the eight pictures were contained in two drops of blood, and ranged in size from 150 microns diameter to filamentous pieces having a length greater than 1600 microns. We roughly estimate that the total number of large sized agglomerates for this pint of whole blood ( actually 498 ml ) would approximate:
17 X 498 X 8 ÷ 2 = 34,000.
The number of agglomerates below 150 micron size, and the number of stringy filaments, exceeded 100,000 per pint. Although these are clearly visible under a stereoscopic microscope, they do not photograph well and therefore are not shown here. A drop of blood viewed through the stereoscopic microscope conveys much more information than can be obtained with the monocular camera.
If the recipient of a blood transfusion had a chance to view with a microscope the agglomerates in the blood he was about to receive, the number of whole blood transfusions would be drastically reduced. And, we are advised by a physician that the use of plasma instead of whole blood offers no significant advantage because it, too, contains a tremendous number of agglomerates.
The writer has found numerous agglomerates in substantially all ACD whole blood examined, from freshly bottled, to twenty–one days old. And this condition is accepted as a fact by technicians performing transfusions. The plastic container for ACD whole blood generally bears the admonition: "A filter must be used in administration equipment." Moreover, the "Technical Methods and Procedures" of the American Association of Blood Banks* states:
[ * American Association of Blood Banks, 30 North Michigan Ave., Chicago, III. – 1962 Edition (p. 53). ]
"Bank blood, particularly with ACD solution as anticoagulant, has a tendency to form minute masses of fibrin on standing. Such blood is perfectly safe if administered through a filter to avoid the entrance of such particles into the recipient. The finer filters ( i.e., 75 – 200 holes per square inch ) are preferable."
The writer takes definite issue with the statement "Such blood is perfectly safe if administered through a filter," because a filter of 75 – 200 holes per square inch cannot pass blood cells ( which are 8 microns in diameter ), and at the same time, satisfactorily exclude coarser agglomerates. To put this matter in proper perspective, the size of capillaries, through which all blood must pass if it is to recirculate, approximates 8 microns. A 200 mesh screen ( at 46.2% open area ) has an opening of 86 microns; and a 74 mesh screen ( at 52.7% open area ) has an opening of 249 microns. It is therefore evident that agglomerates up to 86 microns size can pass a 200 mesh screen; and agglomerates up to 249 microns can pass a 74 mesh screen.**
[ ** These data are from the W. S. Tyler Co., and represent their stainless steel bolting cloth. If the screen were calibrated in terms of "holes per sq. inch" according to the published standards of the AABB, the above values would be increased approximately tenfold. Monofilament nylon screens also have comparable clear openings. ]
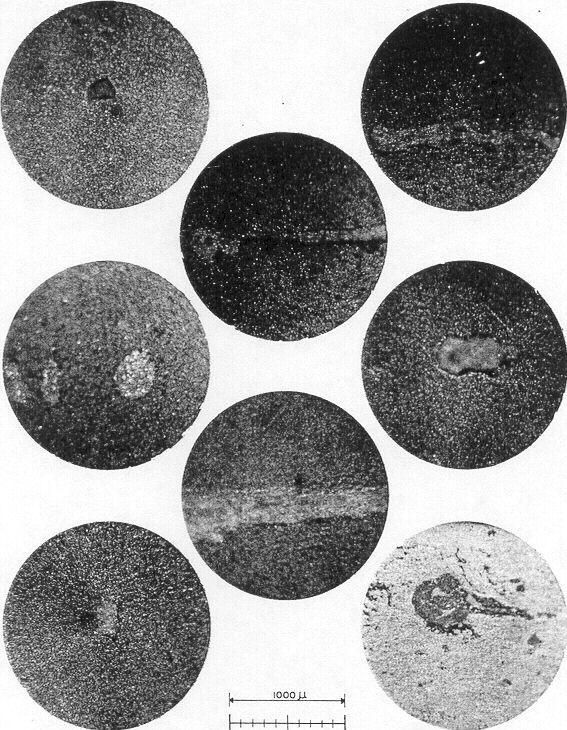
Photomicrographs of massive agglomerates present in two drops of Red Cross bottled ACD whole blood for transfusion. The blood was prepared in accordance with the NIH formula. The concentration of large agglomerates ( as pictured ) was in the range of 34,000 per pint; smaller agglomerates exceeded 100,000.
Embolism must result unless agglomerates which pass the screen can be immediately lysed by dispersive components present in the individual's plasma at the time of transfusion. And the ability of the individual to "dissolve" these particles would seem to be presently unknown. The difficulty in disrupting agglomerates, once formed, is germane to Physical Chemistry, and has been stressed many times by the writer in this text. Concepts to the effect that these agglomerates are quickly lysed by the blood of the recipients cannot be based on demonstrable proof other than the fact that many patients fail to suddenly die as a result of such transfusions. Most anatomists hold that the actual removal of these agglomerates takes place in the lungs. But when one has reached a point where a blood transfusion is indicated, it is doubtful that the lungs are in any condition to cope with the additional load of irritants. This would be particularly true with heavy smokers.
These agglomerates create a hazardous situation, which can never be overcome by the present NIH ( or PHS ) anti–coagulant formula; or by the use of a smaller ( say 9 micron ) screen — because this size mesh would clog immediately.
It is generally agreed that embolisms can cause death. Therefore, when patients die in shock after a whole blood transfusion, it would seem difficult to know whether:
2.) They died from their inability to lyse these aggregates in the transfused whole blood;
3.) Death resulted from mismatching of blood, or other causes.
There is evidence that death following transfusion can be attributed more to embolism and intravascular coagulation, than to decalcification. Col. Hardaway, at Walter Reed Army Institute of Research, published a paper entitled "The Influence of Extracorporeal Handling of Blood on Hemorrhagic Shock in Dogs".*
[ * "Experimental Medicine and Surgery" — Vol. 23, No. 1, January 1965. ]
In his "B" experiment ( involving 30 dogs ), more than 400 ml of blood was withdrawn to lower ( to 40 mm Hg ) the mean aortic blood pressure, from its normal of about 150 mm. The blood withdrawn was treated to prevent coagulation by the standard ACD formula**
[ ** This formula is given above and is a USPHS ( or NIH )
Regulation. it will be found in "Regulations — Title 42 — Part 73 — Biological Products — Public Health Service Publication No. 437, Revised 1964 — Sections 73.300 through 73.306, pp. 46-49." These standards are adopted by the AABB, and appear in their "Technical Methods and Procedures" Revised edition — 1962 — p. 81. The concentration of the three reagents, employing Solution A, is shown in Column 4. If Solution B is employed instead of A, these values will be about 8% less — actually 2640, 960, and 2940 ppm, respectively. ]
—and by heparin. After a period of two hours of this induced shock, the removed blood ( except that used for test purposes ) was transfused back into the original test animals. A filter was employed for these transfusions.
|
Reagent (1) |
Solution A (2) |
Solution B (3) |
Conc. in ACD Whole Blood - ppm (4) |
|---|---|---|---|
|
Tri–sodium citrate ( Na3C6H507 • 2H20 ) | 22.0 g | 13.2 g | 2870 |
|
Citric acid ( C6H8O7 • H20 ) | 8.0 g | 4.8 g | 1040 |
|
Dextrose ( C6H1206 • H20 ) | 24.5 g | 14.7 g | 3200 |
|
Water for injection (U.S.P.) to make | 1,000 ml | 1,000 ml | — |
|
Volume per l00 ml. blood | 15 ml | 25 ml | — |
Five of the 30 dogs died within 48 hours. Autopsies revealed significant intravascular coagulation. However, there were no deaths in his Experiment "C" with four dogs. Here the technique remained the same, except that normal saline was transfused instead of the ACD whole blood, which had been originally withdrawn from the test animals. Moreover, the duration of Experiment "C" was extended to five hours.
In Experiment "C", Hardaway concluded saline was much less toxic than blood which has been in contact with metal, air, or glass. But the absence of fatality could also result from the fact that the saline contained no agglomerates — whereas the ACD whole blood undoubtedly contained many.
I submit that in the case of the five dogs that died in Experiment "B", an interpretation can logically be made that their plasma just could not adequately lyse these agglomerates. Hardaway reports that they died of intravascular coagulation.
The ability of specific blood systems to lyse or prevent the formation of agglomerates, was well demonstrated by Hardaway's Experiments "A-1" and "A-2." In these, and under similar conditions, the injection of heparin into dogs prior to removal of blood actually halved the mortality of the animals so treated.
if, in fact, it were impossible to prevent these agglomerates in whole blood for transfusion, this situation could only be considered "unfortunate." But this is not the case. It can be stated without equivocation that the techniques advocated by the NIH ( or PHS or AABB ) for the collection and bottling of whole blood for transfusion are completely incompatible with known and demonstrable laws of Zeta Potential and are extremely erroneous. It is not surprising to the writer that ACD whole blood is badly agglomerated; indeed, the wonder is that agglomerates are not more numerous.
In the case of open heart surgery, this situation is pathetic from the patient's standpoint. It is widely acknowledged that the death rate is shockingly high among the "late middle–age" group. For most prosthetic valve replacements, it is often a direct function of the pints of blood required for the operation; and the number of hours of cardiac bypass. Even the priming of the pump–oxygenator and filling of the vessels for extracorporeal circulation often presently requires five ( or more ) pints of whole blood.*
[ * We are advised by friends in the medical profession that the present trend in the U.S.A. is to employ plasma instead of ACD whole blood for "priming." Abroad, whole blood is still being used, but in one instance, total priming requirements were reduced to less than one liter. ]
Inevitably, some agglomerates pass the filters, and every agglomerate, which passes the filters if it is not immediately lysed by the patient, must form an embolism.
Cardiologists tell us that for many open heart patients, the operation itself poses the utmost struggle for survival. The imposition of additional risks incurred by the presence of these needless agglomerates**
[ ** Actually it is possible–to–probable that the same reagents now employed would be suitable if they were properly employed, and at their optimum concentrations. ]
is unconscionable. The writer called this to the attention of appropriate Government Agencies in 1965 but he could elicit no interest. A rather impatient reply was received from a director of one of the U.S. Public Health Service medical departments, who stated: "To dispel any lingering fears you may have, we can add the fact that either because of the filtration of the blood when it is given, or because of the ability of the human body to dispose of minute particles which may pass the filter, there is no evidence of ill effects from "agglomerates in Red Cross whole blood", even though more than five million units of whole blood are transfused each year in the United States. You are, of course, correct in pointing out that a number of diseases are associated with intravascular coagulation, but these have no apparent direct connection with transfusions."
The writer rejects this reply as being totally unacceptable. It did not in any manner take into account or even acknowledges the problem. Instead the answer was obviously tailored to suit the exigencies of the occasion. The "exigencies" could only be that the director was afraid and / or unwilling to face the hard facts.
|
Cation or Anion (1) |
Molecular or Atomic wt. (2) |
mEq / l to mg / l (3) |
mg / l to mEq / l (4) |
Normal conc. in Serumm mEq / l (5a) |
Normal conc. in Serumm mg / l (5b) |
|---|---|---|---|---|---|
| Ca ++ | 40.0 | 20.0 | 0.0500 | 5.0* | 100 |
| Mg ++ | 24.3 | 12.1 | 0.0825 | 1.8* | 22 |
| Na + | 23.0 | 23.0 | 0.0435 | 143.4* | 3280 |
| K + | 39.1 | 39.1 | 0.0256 | 4.8* | 187 |
|
| 155.0 | 3589 | |||
| Cl – | 35.5 | 35.5 | 0.0282 | 104** | 3700 |
| HCO3 – | 61.0 | 61.0 | 0.0164 | 27** | 1650 |
| HPO4 – – | 96.0 | 48.0 | 0.0209 | 2.0** | 96 |
| S04 – – | 96.1 | 48.0 | 0.0209 | 1.0** | 48 |
|
| 5494 | ||||
|
| 9083 | ||||
| Protein Anions | — | — | 18.0** | — | |
|
Other Organic Anions (Lactate and Pyruvate) | — | — | 3.0** | — | |
|
| 155.0 |
| |||
[ * From Nutritional Aspects of Cardiovascular Diseases
Baiusz – Lippincott – 1965. ]
[ ** From Reference 11-10. ]
|
| Concentration in mg / l | |||
|---|---|---|---|---|
| Element | Blood | RBC | Plasma | Serum |
| Aluminum | 0.15 | 0.07 | 0.46 | — |
| Bromine | — | — | — | 0.01 |
| Copper | 0.93 | 0.75 | 1.11 | — |
| Fluorine | 0.28 | 0.27 | 0.28 | — |
| Iodine total | 0.07 | — | — | 0.07 |
| Iron | 0.48 | — | — | — |
| Lead | 0.29 | 0.57 | 0.03 | — |
| Manganese | 0.13 | 0.19 | 0.08 | — |
| Silicon | 0.50 | — | — | — |
| Tin | 0.22 | 0.26 | 0.04 | — |
| Zinc | 8.8 | 14.4 | 3.0 | — |
[ * From Ref. 11-10 ] — Periodic Table of Elements —
|
| Approx. Avg. mg / liter of Plasma* |
|---|---|
| 1) Glucose (fasting-serum) | 1,000 |
| 2) Lipids-total | 5,300 |
| 3) Neutral Fat | 1,400 |
| 4) Phospholipids | 1,600 |
| 5) Cholesterol | 1,500 |
| 6) Fatty Acids-total | 3,100 |
| PLASMA PROTEINS | |
| 7) Total | 74,800 |
| 8) Fibrinogen | 2,800 |
| 9) Albumin | 52,000 |
| 10) Globulins | 20,000 |
[ * Unless otherwise noted. ]






"Control of Colloid Stability through Zeta Potential"
Thomas M. Riddick's Chapter 14, part 1 — " Dynamic Systems "
Static images help us understand, but we live in a Dynamic World & need to view it that way.
CHAPTER 22
The application of basic concepts of Zeta Potential to cardiovascular disease
Thomas M. Riddick's personal experiences with a cardiovascular condition.
( Sometimes you should read the last chapter of a book first. )
Chapter 19 — Dilatancy, Thixotropy, and the Double and Diffuse Layers
Excerpts and Important Material — Glossary
Riddick's Suggested Reference Mateial
Dr. T.C. McDaniel — "Using Zeta Potential as a Healing Tool"
Using Hydroponics to Understand the Earth's Life Processes
On the Atomic Level
Site Link List - Hydroponic Reference Center - Hydroculture Salts
The Tortoise Shell "Science of Health" Newsletter
— Putting an End to Disease on Our Planet —