The Heat Pump
Elements of simple compression system.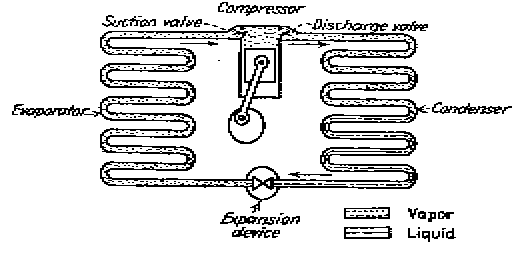
143K audio *.wma file
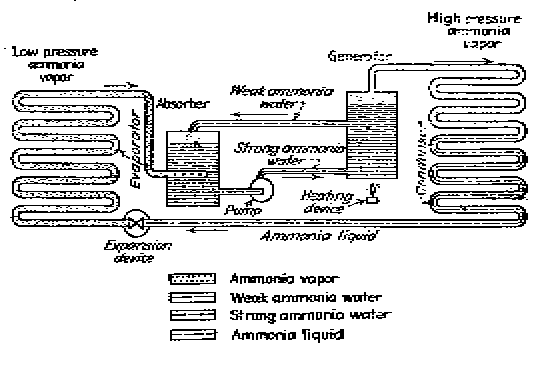
Elements of simple absorption system.
In the ordinary sense, heat is the measure of vibration between atoms in a
molecule. At absolute zero, there is no inter–atomic motion. No motion,
no heat; Zero; Our " Reference Point "! This is where we
mathematically place our "Zero" [ 0 ].
Therefore, there is no such creature as cold. Cold is a slang term and
should not be used in science. Something can be cool, which is a relative
term, when referenced, but, Cold is not an Entity, like Heat is !
So, what about those other zeros [ 0's ] you have heard about ?
Those are for the weather man. He could use the Kelvin scale, which uses centigrade divisions and starts at the " No Motion — Absolute Zero " point.
Or, how about using the English Fahrenheit, scale divisions ?
Most of you know that there is more to the Temperature Story than this,
but, ...
So, Temperature can be defined as how fast the dance is going; the Tempo.
Ops, this quality doesn't equate directly to the thermometer !
So, Temperature Does Not tell you How Much Heat there is !
At any given temperature, a cubic centimeter of iron has more kinetic heat energy, than a cubic centimeter of Aluminum.
Different materials can hold different amounts of heat. The amount of heat that a material can hold is Specific for that material. We refer to it in the reference books as .... Yup .... " Specific Heat ".
Specific Heat is largely the result of Density. The number of dancers on the dance floor. You all know how Hot, a crowded dance floor can be.
When we want to know " How Much Heat ", we have, or need, we must consider the temperature in relation to " Specific Heat ", and weather-or-not there is any " Change of State Energy " involved.
" Temperature ", measured in degrees; multiplied by " Specific Heat ";
Darn ! Here is where a nice simple concept like Heat, gets messed up.
And, what kind of scale do we need for weight ?
Temperature — Degrees Kelvin
Weight — Grams
Length — Meters
Volume — Cubic Centimeters
Pressure — Dynes / cm2
Temperature — Degrees Fahrenheit
Weight — Ounces; Pounds; Tons; ...
Length — Inches; Feet; Yards; ...
Volume — Ounces; Quarts; Gallons; ...
Pressure — Pounds / in2; Inches of
Hg
That which is inappropriately called Radiant Heat is a sub-set of
LIGHT.
multiplied by " Weight ", measured in ...
Which " Degrees " are we using for temperature ?
For our measurements, do we use ...
Systems of Measurement
Needed to Engineer Atoms
*Units Used by Most Reference Books
Metric
Vs
English
HEAT — Calories [ Cal. ]
*HEAT — British Thermo Units [ Btu. ]
Radiant Heat ? Bad Wording !
( Just another little problem with our Scientific
Language. )
It is possible to "Pump Heat" from one place to another, even if the destination is at a higher energy level than the source. This technology is derived from the Science of Thermodynamics.
Lucky for us, Mother Nature has established a consistent set of rules or laws that remain constant throughout the ages. Various people have discovered these "Laws" through observation and experimentation, and often these relationships are named after them.
"The volume of a gas varies inversely as the pressure, provided the temperature remains constant."
This means that, if a certain quantity of gas has its pressure doubled, the volume becomes one half of the original. Conversely, if the volume becomes doubled, the gas has its pressure reduced by one half.
This law is based on absolute temperature and absolute pressure.
Absolute Zero = – 460 degrees Fahrenheit or – 273 degrees Centigrade.
Absolute Pressure is based on the pressure of outer–space being equal to 0. The average "absolute pressure" at sea level is equal to 14.7 pounds per square inch.
Let me repeat! Each gas behaves as if, it were the ONLY gas in the room. This is a Very Important Point, as we will quickly experience, as we study how Water moves about in your "Tortoise Shell".
Substances always exert a pressure upon the surfaces supporting them. Therefore, the Force of our Planet's Atmosphere, is the result of accumulated partial pressures.
PA = absolute pressureNow that we have the basics, we can learn how to move heat around.TA = absolute temperature
VSI = volume in square inches
P X V = a constant number
Pa X Va = Pb X Vb ( at a constant temperature )
P1 X T2 = P2 X T1 ( at a constant volume )
V1 X T2 = V2 X T1 ( at constant pressure )

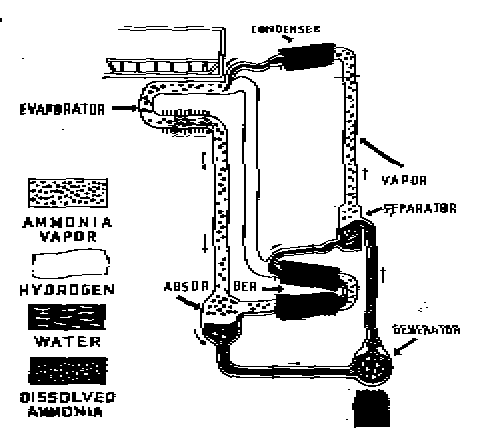
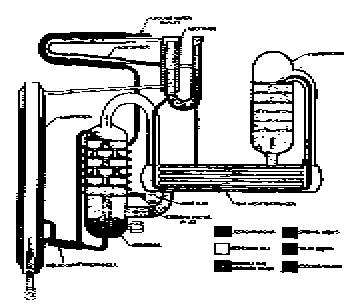
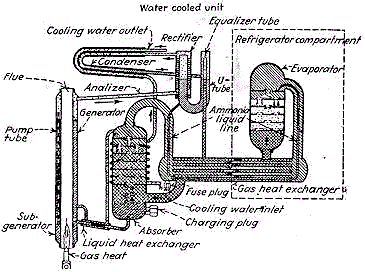
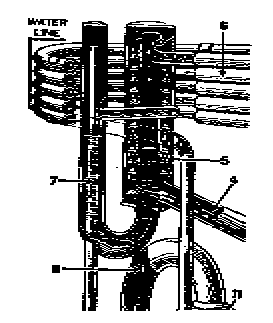
Elements of simple absorption system.

The action of this system depends also, on Dalton's Law which states that two gases within a closed container exert a total pressure equal to the sum of the individual (partial) pressure of the gases. Also, according to this law, the temperature at which a liquid boils, or at which a gas condenses in an enclosed space corresponds to the partial pressure exerted by that gas.
The ammonia / water absorption refrigeration system can be used with almost any source of heat which produces a temperature of 300 degrees F. or more.
The operation of the ammonia / water heat pump depends on three different cycles which take place simultaneously and are repeated continuously:
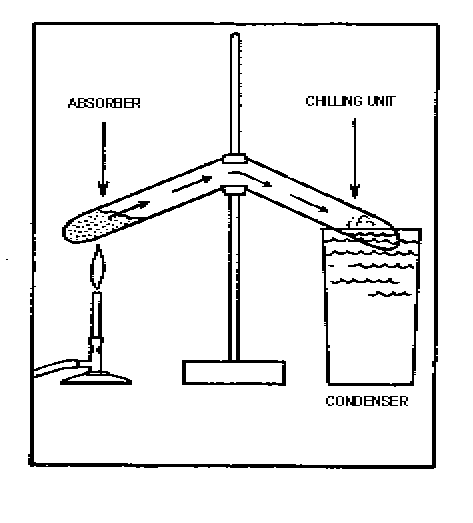
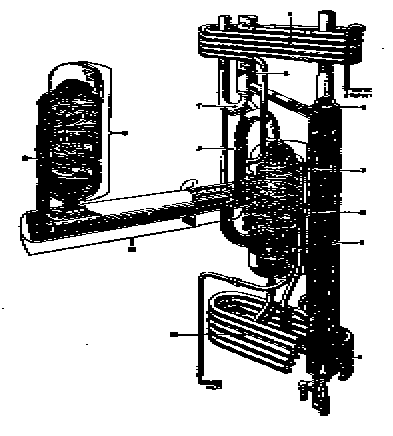
Elementary solid absorbent cycle
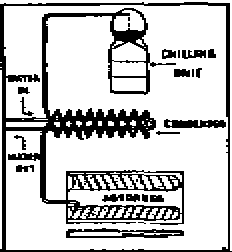
Various solids also can be used to absorb and release a refrigerant.
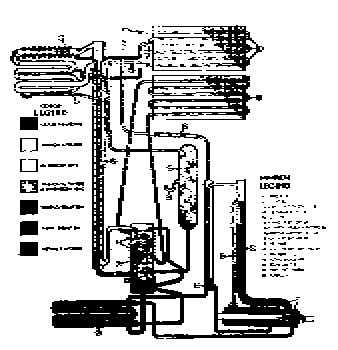
The Faraday Cycle
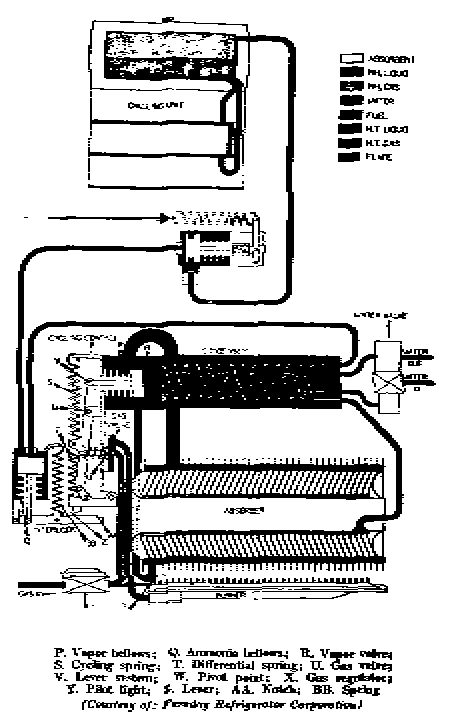
The air-cooled Servel refrigeration cycle
The complete Servel Design
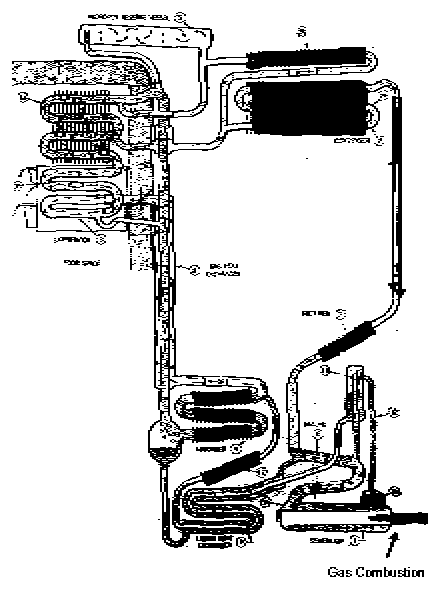
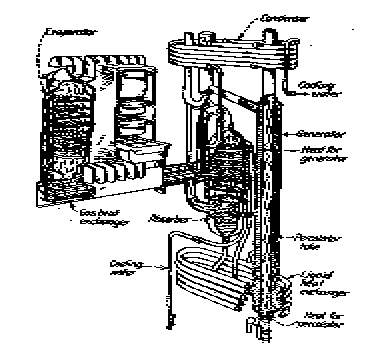
The Electrolux air-cooled cycle
The 1935 model Electrolux air cooled unit has a charge pressure of 380 lb./sq.
in.; 8 cubic feet of hydrogen; 1 pint liquid ammonia and 2 pints of water.
The Gibson shelf and condensing unit

The Gibson unit used sulphur dioxide as a refrigerant.
( Very, Very Toxic ! )
References
Modern Refrigeration and Air Conditioning
Andrew D. Althouse and Carl H. Turnquest
The Goodheart - Willcox Co. Inc. Chicago, 1956Modern Electric and Gas Refrigeration
Andrew D. Althouse and Carl H. Turnquest
The Goodheart - Willcox Co. Inc. Chicago, 1933Household Electric Refrigeration
Wostrel and Praity
Mcgraw - Hill, 1948
Site Link List - A New World of Possibilities
The Tortoise Shell Life Science Puzzle Box Front Page