BIOlogical TRANSmutations
By
Professor C. LOUIS
KERVRAN
Member of the New
York Academy of Science
Director of
Conferences of the Paris University
Member of Conseil
d'Hygiene de la Seine
English Version
Michel Abehsera
© 1989 M. C. Escher c/o Cordon
Art – Baam – Holland
Jacques de Langre,
Ph. D. Editor
Happiness Press
Magalia, California
Biological Transmutations
and their applications in:
Chemistry,
Physics, Biology, Ecology, Medicine, Nutrition, Agronomy, Geology.
C. Louis Kervran 1901-1983
Nominated for the 1975 Nobel Prize in Physiology. Nominator:
Hiroshi Maruyana M.D. Ex-professor, Faculty of Medicine, Osaka University.
Nomination supported by Professor L. Tanon, President of the Superior Counsel
of Hygiene of France. In a last minute change, the prize went to someone else.
Other Works by C. Louis
Kervran
ORIGINAL FRENCH EDITIONS:
Safe Limits of Alternative Tensions. . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . RGE Paris 1937 Resistance of the
Human Body to Electricity. . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . Lahure 1936 Electrocution at Low Tension is avoidable '. . . . . . .
. . . . . . . . . . . . . . . . . . . . . . . . . Masson 1939 Aberrant
Metabolism and Biological Transmutations
Revue Generale des Sciences Pures et
Appliquees . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1960
Biological Transmutations, Abnormal metabolisms in Nitrogen Potassium
and Magnesium. .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . . . . . Maloine 1963 Natural Transmutations, non Radioactive, A New
Property of Matter . . . . . . . . . . . . . . . . . .1963
Low Energy Transmutations, Synthesis and Developments. . . .
. . . . . . . . . . . . . . . . . . . . . . 1964 Proofs Relative to the
Existence of Biological Transmutations, In Biology, defeat of
the Lavoisier
Law of Matter's Invariability. . . . . . . . . . . . . . . . . . . . Maloine
Library SA 1968
Biological Transmutations in Agronomy, Conferences at the
National Agronomy
Institute. . . .
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .
. . . Maloine Library SA 1970
Geological and Physical Proofs of Low Energy Transmutations. . . . . . Maloine
Library SA 1973 Biological Proofs of Low Energy Transmutations, with added note
of O. Costa de Beauregard (Theoretical Physics) . . . . . . . . . . . . . . . .
. . . . . . . . . . . . . . . . . . . . . Maloine 1975
Biological Transmutations and Modern Physics . . . . . . . .
. . . . . . . . . . . . . . . . . . .Maloine 1982
All of the above
books and publications are in French.
ENGLISH
TRANSLATIONS:
(The present volume
combines three original French works)
Biological Transmutations (From the French 1966 Edition). .
. Crosby Lockwood, Glasgow 1980 "Bread's Biological Transmutations"
in collaboration with Jacques de Langre, in English,
Happiness Press Out of Print, see update below 1978
"Good Bread, Evil Bread, Baking by the Biological
Transmutations Principle"
An expanded and revised edition of
the 1978 title above. . . . . . . . .Happiness Press 1988
Published by Happiness Press
Jacques de Langre, Executive Editor
4351 Wycliff Way, P.O. Box DD
Magalia, California 95954
Printed in Hong Kong
INTRODUCTION TO
THE SECOND EDITION
Fate and circumstances! In the fall of 1972, a friend sent
to me, from Old Mexico, a copy of Michel Abehsera's translation of Professor
Kervran's Biological Transmutations. During the following years, so long as the
book was in print, I bought dozens of copies for friends and others whom I
thought might share my fascination with this incredible hypothesis. The
reactions were mixed, and challenging: Can it be proven? What does it mean?
Impossible, of course! It would be wonderful if true. Being concerned with both
plant and animal nutrition, I became convinced the idea should be put into
practice. To de this, at least in an acceptable way, meant proving the
hypothesis by repeatable experiment, not an easy task, I was to learn. Early
on, my DVM friend, John Whittaker, said: "Why try to prove it? Biological
Transmutation just is, and it is just beautiful. Later, in a private conversation, James Lovelock, internationally
known atmospheric chemist, remarked that, in reality, it is practically
impossible to prove anything whatsoever. And besides he said, "... even if
you did prove biological transmutations to yourself and to me, no one else
would believe it!" My curiosity went out of control. In the fall of 1981,
I began a serious effort to research Professor Kervran's works. Progress was
slow and discouraging. Finally, in August 1982 I tracked down Chris Bird. I
knew that he, with Peter Tompkins, had written The Secret Life Of Plants, a
1972 non-fiction bestseller containing a chapter about Professor Kervran. My
assumption that he knew this French genius personally was correct. Chris sent
me a 2,501 page file, his years of correspondence, a typescript of his
translation of Kervran's 1973 book, Proofs in Geology and Physic of Weak Energy
Transmutations, and his partial translation of the 1975 book, Proofs in Biology
of Weak Energy Transmutations. But this was only the beginning.
Professor Kervran died February 2, 1983. Just three months
prior, his last book, Biological Transmutations and Modern Physics, his
"swan song", had been published by Maloine, Paris. I had this book
translated and the typescript has now been partially edited. Subsequently,
Chris retrieved from Europe hundreds of pages of Professor Kervran's worldwide
correspondence together with an incredible number of published and unpublished
papers and articles, including copies of all of Kervran's eight published
books. In the fall of 1984, Chris translated the entirety of the 1970 book,
Biological Transmutations in Agronomy, a series of lectures given by Kervran at
the National Institute for Agronomy in 1969. He also translated hundreds of
pages of correspondence, and our own translator finished this part of the
project.
Professor Kervran's archive file now contains over 5,000
pages. The accumulation of related materials seems endless. I have resisted a
number of requests to edit and publish the three fully translated books.
Together, Professor Kervran's many books display a valuable progression of
knowledge, but individually they do not do justice to the man or his ideas.
Michel Abehsera's translation avoids this problem by summarizing Professor Kervran's
pre-1970 position. A major book is in the formative stages, a book that will
not only detail Professor Kervran's saga, but also compare and contrast his
observations and theories with those of others. In addition there are new
perspectives in both physics and biology to be integrated into this fascinating
story.
The biological transmutation hypothesis cannot be treated as
an isolated subject. It questions the very nature of substance, a subject
philosophers and physicists alike continue to ponder. I remain convinced there
are elemental transmutations in living systems, and further, that living
systems may very well also create elements. Curiously, in the final analysis,
it becomes difficult to distinguish between transmutation and creation.
To my best knowledge, of the thousands of experiments performed
to prove or disprove Professor Kervran's theory, none are conclusive. This is
to say, in the view of normal science, each and every experiment contains a
flaw, some factual, some simply a loophole through which doubt can enter and
invalidate the data. Fundamental problems remain to be resolved in designing a
scientifically acceptable crucial experiment.
First, there is the problem of measuring the before and
after composition of a singular living system. To satisfy the scientific mind,
absolutely reliable data can be had only by killing the system. To analyze one
killed system and compare the data with that of an apparently identical living
system, killed after an experimental period, leaves the data open to criticism,
justified or not.
Secondly, living systems are open systems, and it is
extremely complicated, if not impossible, to totally isolate them under the
laboratory conditions required for accurate monitoring. Further, all methods of
isolation create an unnatural environment for living systems. For example, in
laboratory experiments with oats, the culture water must be free of all
chemical elements, absolutely pure. This "laboratory water" is dead
water, not the same as the living water found in nature, a problem with which
Professor Kervran was much concerned. In addition, in nature, plants are
symbiotically dependent on bacteria. It is apparent that in all the extensive
experiments with oats the culture water was "contaminated" with
bacteria. Thus, these were not pure plant experiments, but experiments with
plants and bacteria. No doubt, the kinds and quantities of bacteria present in
the culture is a significant if not crucial factor.
Thirdly, biological processes are at best periodic, and at
worst fluctuating. This requires an experimental protocol, which allows for
cosmological influences. Even if the scientific community was tolerant of such
parameters, which allude to astrology, they make the uniform replication of
experiments ever so much more difficult.
Living matter exhibits a number of self-evident properties
such as evolution, symbiosis, and bacterial pleomorphism phenomena, which
remain beyond science, facts without theory. We now have an enormous collection
of facts about evolution but the phenomenon resists scientific explanation. We
do no have an acceptable theory of evolution. Unbelievably complex symbiotic
relations between different kinds of living systems are recognized and
cataloged, but a theory of symbiosis is so elusive some biologists now question
if there is really a difference between symbiosis and parasitism. Bacterial
pleomorphism has been recognized by a few biologists for over one hundred
years. Extensive hard evidence for this phenomenon has been accumulating since
1960, but the facts are only self-evident, without theory .... not
scientifically acceptable!
It is obvious to my mind that biological transmutations must
be included in the list of "self-evident-facts-without-theory"
properties of living matter. My friend, Dr. Whittaker, was prophetic when he
said, "... biological transmutation just is." The phenomenon cannot
be explained by normal physics or chemistry. This is not to say the structure
of science cannot be evolved to a point where such properties of living systems
can be utilized in practical applications rather than excluded with prejudice.
Though much has been learned during the fifteen years since
this translation of Professor Kervran's work was first published, it remains an
excellent introduction into the complexities of a fascinating and elusive
natural phenomenon.
Jacques de Langre is to be complimented for his initiative
in having this important book republished.
John W. Mattingly
Affiliate Staff Member
Department of Philosophy
Colorado State University
Fort Collins, Colorado 80523
February 18, 1987
CONTENTS
Foreword
Introduction
to the Biological Transmutations
I Aberrant Observations
II Potassium
III Sodium- Potassium
IV Calcium
V Potassium-Calcium
VI Production of Calcium from Silicon
VII Magnesium (Endogenous Production)
VIII Magnesium-Calcium Relation
IX The Link of Magnesium with Calcium and
Phosphorus
X Phosphorus
XI Aberrant Metabolism of Some Living
Organisms
XII Nitrogen
XIII Sulfur
XIV Chlorine
XV Manganese and Iron
XVI Variations of Minerals in Dried Fruits
XVII An Interpretation of an Analysis Made on Rye
Grass
XVIII Transmutation of Radioactive Wastes
XIX How to Make Experiments with Biological
Transmutations Successful
XX Agriculture
XXI Nutrition
XXII Medicine
Epilogue
Bibliography
John W. Mattingly Affiliate Staff Member Department of
Philosophy Colorado State University Fort Collins, Colorado 80523
FOREWORD
This English edition of the works of Louis Kervran is
intended for everyone: scholar, layman, or college student. It could have been
directed toward a scholar's mind exclusively, presenting itself to him in cold
scientific terms, but this would have slowed down progress. If, in Mr.
Kervran's words, scientists "have made of science another job," it
would not be ethical to present it to them alone. The problems of ecology,
medicine and nutrition, and the alarming rise of radioactivity are too acute to
be dealt with solely through academic channels. It is within everyone's ability
to comprehend the biological transmutations as long as there is a desire for
true knowledge. To understand the biological transmutations requires nothing
more than to cast aside all rigid thought while studying them. Transmutation is
no more and no less than a reality, which teaches us about change. In change we
find life, and by change we create life. Our only constant is our goal of
becoming Man.
The principles of the biological transmutations affect every
phase of our existence. They are already being applied without patent or
license by industrial, dietetic and pharmaceutical products based on Kervran's
research. Transmutations are now recognized in medicine. They have opened the
door to new treatments and therapeutics for reputedly "incurable"
diseases. There are solutions already projected for curing arteriosclerosis,
rheumatism, excessive arterial tensions, decalcification, kidney stones, hormonal
deficiencies, etc., in a natural way, without danger to the patient.
Agronomists are already practicing Kervran's findings on a large scale.
Dietitians are the biggest beneficiaries, because dietetics is too close to
man's body and soul to remain an academic and isolated science. In accordance
with this law of the change of elements, which Louis Kervran uncovered, there
is now a movement by hundreds of thousands of people to eat in a natural way.
The U.S.A. is ecologically in bad shape. Only through the understanding
and practice of biological transmutations can this alarming problem be solved.
The land is not only polluted but is in some parts barren. Heavy chemicals have
killed it. No ecological problem can be solved on the surface; it can only be
remedied as its name indicates: ecologically. This means that it can be done
only through natural law. Ecology starts with man, mind and body. If his body
is clean, his environment will be clean, naturally. If our tastes are
sensorial, if our measure of happiness and health is comfort, then ecology is
relegated to a mere science with man as a hopelessly retarded child who must be
dealt with by caresses and long walks for a year or two or three, until the end
of his invalid life. Ecology is often synonymous with attractive environment.
Cans of beer are removed from beaches; hands are cleaned; everyone returns home
satisfied. That is merely a good cleaning job.
What about man? What about his body and mind? If the food
being consumed does not change, the problem of ecology will worsen. If
scientists do not change their minds about nutrition, man, a biological part of
this earth, will soon be extinct. Intestines must be healed, since they are as
polluted as our streams. The populace must be educated to choose food that
does not come canned, bagged in plastic, or chemically treated. From the need
of the consumer, from this need only, can the ecological problems be considered-and
solved. From a good earth one regenerates one's health. Our blood is but the
product of an agent, the earth. The biological creates the physiological. Has
this become a secret, or has man become so mechanical, so enslaved to fast
relief in any possible way, that he has become accustomed to the "miracle
pill"? His pain is relieved in seconds. This is proof enough for him to
believe in the "scientific way." But that is not true science.
In a letter to the author of this English version, Louis
Kervran wrote: "I hold the same conception as you do: if one desires to
bring about a deep change in science, it is not to the high scientific spheres
that he should address himself, but to the masses. Not being narrow-minded like
the specialist, they can see the synthesis of things and not just their
details. This is how I have been progressing in this world during the last ten
years ... But one should not be so absolute in judgment as to say that all
scientists are narrow-minded. I have found great support among known
personalities, but they are a minority. They are lost in the crowd all cannot
vanquish the obstacles and inertia created by the too many. Science has become a petty job. Fifty years
ago there were a few thousand men of science; now it is by the hundreds of
thousand that one counts them, so that the average level of quality has greatly
diminished."
What Mr. Kervran means is that man has become mechanical in
his thinking and doing. The physiological, which is what we "are,"
and the biological, from which our daily nourishment comes, are but a shadow of
what man used to be when he was free. What is freedom if it is not to be free
in every way, from our most minute cell to our most expansive dreams? He is free
who can afford to let the interactions between cell and spirit take place in
the most harmonious way. There is no freedom in intellect. "Freedom"
of that sort lasts for the duration of a though of an act. To be truly free is
to be able to establish peace between all opposites within us. That is at least
the beginning of freedom Mr. Kervran, with his discovery of biological
transmutation, has given this chaotic and scientific world the Alladin's Lamp
by which we can save ourselves and our diseased earth. From mechanical men we
will raise ourselves to physiological and spiritual: beings. That which is
mechanical does not believe in change, nor in beauty, nor even in man. The
mechanical mind lives within the hour. If it projects an idea for the future, it
is but a steel-like construction erected out of stress, a protection from the
"invaders". The mechanical man is afraid. He thinks the enemy is
outside of him, so he protects himself a bit more every day until he loses tl1
natural immunity he inherited from this just world.
With biological transmutations we are taught a lesson about
freedom. We learn that an element is free to become another when it meets its
opposite. It travels from one state to another procreating newborn atoms. It is
the hope of Mr. Louis Kervran and this author that biological transmutations
will be applied to the fullest extent in the U.S.A. and in the whole world.
In 1799 the French chemist Vauquelin was so intrigued by the
quantity of lime excreted every day by hens that he decided to put a hen in a
cage and feed it oats exclusively. Having measured the quantity of lime that
was present in a pound of oats, he gave the oats to the hen. When the grains
had been eaten, he analyzed the quantity of lime excreted through the eggs and
fecal matter. The hen was found to have excreted five times more lime than
it had taken in the food. Vauquelin concluded that lime had been created,
but he could not determine the cause.
In 1822 Prout, an Englishman, was the first to clearly
define the problems of the transmutation of elements. He systematically studied
the increase of limestone (a compound consisting chiefly of calcium carbonate)
inside an incubating chicken egg, proving that limestone is not contributed by
the shell.
In 1831 the Frenchman Choubard let watercress seeds germinate
in an insoluble dish (sand, glass, etc., washed with acids, rinsed with water,
heated). He verified that the young plants contained minerals, which had not
existed in the seeds.
Others followed. In 1844 Vogel experimented with watercress
seeds placed under a large bell jar. Keeping the air "analyzed," he
added a nutritive solution containing no sulfur whatsoever. After their
germination he analyzed the young plants, finding that they contained more
sulfur than the seeds from which they stemmed. This phenomenon remained obscure
to Vogel, who concluded that either sulfur is not a simple body or there was
an unknown source of sulfur.
A few years later Lauwes and Gilbert considered the weight
variation of ashes during vegetation. They observed, in analyzing the ashes, an
inexplicable variation in the amount of magnesium. In 1875 von Herzeele went a
step further by verifying a weight increase in the ashes of young plants
stemmed from germinating seeds. He made a culture without soil in a
well-studied medium. Later on he carried out experiments related to Vogel's
earlier study of the weight variation of magnesium, already considered by
Lauwes and Gilbert. Von Herzeele then concluded that there was a transmutation
of elements.
He seems to have been the first to research the origin and
destination of an element. His remarkable work remained without echo in the
scientific world. In 1950 Hauschka took it out of the dark to publish von
Herzeele's findings in one of his books.
Branfield, Lakhovsky, Spindler and Freundler, among others
followed. None of them arrived at clear conclusions.
Baranger, chief of the laboratory of organic chemistry at
the Ėcole Poly technique in Paris, became acquainted with von Herzeele's
work. In 1960 he published the first results, handed to him by A. Spindler, of
the variations of phosphorus and calcium in germinated seeds. Baranger
concluded that a transmutation of elements had occurred, but his research, like
that of his predecessors failed to show the mechanism by which the discrepancy
was produced.
It was in 1959 that Louis Kervran started publishing his
discoveries. At about that time the coincidental works of the above scientists
became known to him. In the summer of that same year Mr. Kervran came to a
conclusion concerning his many years of systematic research. He did his best to
prove his conviction to the scientific world, a conviction that none before him
had been able to put down in clear formulas. Having waited for years,
witnessing thousands of convergent analyses, he succeeded in demonstrating that
not only molecules but (also) atoms themselves can be transformed. He verified
that there is transmutation of matte from one simple body to another, from one
atom to another.
All the proof was presented, yet Kervran encountered violent
opposition from skeptical people who were reluctant to accept something new. On
the other hand, warmest congratulations and encouragement poured in from very
great personalities of science who now saw light in what had always been
obscure to them. Already in 1959 Kervran had found solid help among those
having true scientific spirit.
In July 1960 a French scientific review published an article
on Louis Kervran. That was enough for the public to know what many scholars had
refused to see. Magazines, radio and television gave millions the chance to be
informed about the "transmutations." It was not until 1962 that
Biological Transmutations was published by Librarie Maloine. In a matter of a
few months, the first edition was sold out and reprinted. A third one was to
follow two years later. Successively came Transmutations Naturelles,
Transmutations à Faible Energie, Preuves Relatives à l'Existense des
Transmutations Biologiques, and Transmutations Biologiques en Agronomie.
In the preface of Transmutations Naturelles Jean Lombard, a
geologist of worldwide reputation, wrote these words: "The true workers of
science, who are always ready to welcome new suggestions, sometimes ask
themselves if the greatest obstacle to the progress of science is not bad
memory on the part of the scholars; they wish to remind the latter that some of
their predecessors were burnt because they proposed 'interpretations' which
have now become foremost truths. If pioneers of science were still being
burnt, I would not give much for Louis Kervran's skin."
In January 1963 a group of scientists listened to Louis
Kervran. After that meeting a report was written by Fischoff, saying: "We
are convinced that there is here a series of observations and phenomena of the
highest importance for the progress of our knowledge in physics, biology,
geology, cosmology, etc. ... The great merit of Louis Kervran is to have felt
strongly that there was something strange in the facts he brings to us and that
there was a need for something 'new' to explain it. He obstinately followed an
idea and patiently, for many long years, accumulated facts, observations, and
results – having no apparent link – at last assembling all these converging
facts and ideas to form a daring hypothesis, solid and thrilling. Daring, for
it appears to be opposed to the classical conception of nuclear physics and
biology. Solid, for the invoked and observed facts are very many, sustained by
an infallible reasoning argument. Thrilling, for it opens new perspectives and
horizons in biology, medicine, energetics, physics, cosmogony, etc."
Twelve years have passed since Biological Transmutations became
public. The debate of the times continues. France is now divided into two
camps, those for Kervran and those against him. English scientists have invited
Louis Kervran to give a series of lectures and demonstrations. Scientists from
Switzerland, Italy and Belgium have already heard him and are starting to put
transmutations into practice. Russia contacted Kervran. All books about
transmutations have been translated into Russian, but not yet on a commercial
scale. They can be consulted now in the Moscow Library. Russian scientists are
now organizing a congress exclusively dedicated to transmutations, to be held
in 1973. The Chinese Embassy officially and insistently invited Kervran many
times to go to Peking for three months to give Chinese scholars a chance to
learn about his work. Nine years ago the Dow Chemical Company invited Kervran
to come to the U.S.A. Mr. Kervran, however could not honor the invitation at
that time.
* * * * *
After I had finished the present book and had written the
major part of this Foreword, I went to see Mr. Louis Kervran in Europe to make
corrections. What I had done was to put all his book into one. It was not an
easy task. Choosing the most essential parts was the first difficulty, for
everything seemed essential. All other difficulty kept arising at almost every
sentence. Kervran's statements were directed at French scientists, who do not
as for as much precision as their American colleagues do. My problem then was
to remain loyal to the text while rendering it in manner suited to the American
scientific mentality. In other words, what was required was a first-class
writer who reasoned as well as he wrote, who was thoroughly familiar with
English an French, chemistry, biology, nuclear physics, etc., and who was
flexible enough to trust the concept of transmutation as a "new
science."
If I ever chose myself to do the present work it was not
because I am that kind of a writer, but simply because I could not find anyone
even half-qualified to do it. There are not many scientists who know French
well enough to decipher Kervran's texts. I reasoned and convinced myself thus:
this is a new way of looking at science; it is up to the reader – whoever he
may be, scientist or layman – to go ahead and grow with it. My role will be
limited to that of a catalyst.
This I may have done well, but I humbly declare myself
incapable of doing what really ought to have been done. Being busy with
studying and teaching, I could afford to spend only six months on the book
(twelve hours daily). Truly it needed another six months. In short, this book
needed a man richer than I in all respects, one who would have spent a year or
two at his desk thinking and working only on the transmutations.
This book is not a textbook or something to use in the
laboratory. Its sole purpose is to stir the minds of those who are still truly
concerned about this earth ecologically. I would never finish if I were to cite
all the possible applications of the biological transmutations. Of special
importance is the place they will occupy between the scientist and the
metaphysician. So far there has never been a direct dialogue between the two,
because of the differences in language. Now I believe their meeting is
possible, for the biological transmutations teach us about the movement of
life. From this movement the scientist will see that science can no longer
limit itself to the study of the physical alone, for movement also implies
elevation toward the metaphysical, not entropy. From this same movement,
animating the invisible elements, the metaphysician will learn that life is
worth studying in its most singular aspects. He will discover in the minute
that which he always knew, that life is a continual renewal of self and cell.
I would like to end this foreword by presenting, in a few
words, Mr. Kervran. I had expected to meet a scientist with whom I would
discuss – as men usually do when they meet – philosophy. But things did not
happen as I anticipated, for he showed himself such a dragon in science that
nothing but science was discussed. Mr. Kervran knew his subject well; he seemed
to have read all the scientific books and articles published all over the
world, to know the work of every living scientist. And when I told him that he
had given to science a new direction and hope, he answered, his face growing
red, "I simply pointed out what has always existed."
The chapters of this book have been placed in the best
possible order for the reader who is unfamiliar with the biological transmutations
to become acquainted with them.
The reader will perhaps meet some difficulties which I
myself met upon first reading Mr. Kervran's books. For this reason I advise
him to read this book more than once, or, if he wishes, to go directly to the
chapter of his choice (Medicine, Agriculture, etc.). In this manner he will learn rapidly where the biological transmutations
can be applied.
May this book be the instrument of a new era in which men of
good will and understanding bring justice and peace on earth.
The English and often the sense of the following text were made
clear by Susan Hatsell. Robert Jones and Hinda Zweig made pertinent remarks
contributing greatly to the clarity of many difficult parts.
For all these I am grateful.
M.A.
On incandescent stoves
In the elementary school classroom in the borough where I
lived, we were heated by a rudimentary stove made of cast iron. There was a key
on the pipe to regulate the draft – or we could push or pull the ashtray. Old
oak was mainly used. When the wood caught fire, the stove very quickly began to
"snore" and became red. Then everyone complained of headaches. That
is why an "officer in charge" was appointed to turn off the key or
push the ashtray when the stove started growing red.
The headaches, the teacher told us, came from the carbon monoxide
emitted by the stove at the red-hot point. At school, however, the belief was
that slow combustion gives off carbon monoxide (CO), while fast combustion
produces carbon dioxide (CO2),
which is less dangerous. We were advised against sleeping in a room having a
stove with a slow draft.
I couldn't make up my mind. If the stove becomes red, it is
precisely because it has a good draft system, a very good one, and there should
be no formation of carbon monoxide. All the explanations given to the questions
I later asked my teachers were so little convincing that this mystery also
stayed in my subconscious.
The scientific answer is this: red-hot cast iron becomes
porous and allows the CO within the stove to seep out instead of leaving by way
of the smoke pipe. If I objected that there could not then be carbon monoxide
from a fast, complete combustion, the reply was that CO2 went through the red-hot cast iron,
became rich in carbon, and then became carbon monoxide. This meant that the
cast iron would eventually lose its carbon! I have never seen a few hundred
grams of coal (approximately 40 grams per kilo of cast iron) disappear from a
cast iron stove to produce a stove of steel! These hundreds of grams of carbon
would have burned quickly! Even if cast iron is porous when incandescent, I do
not believe carbon monoxide is produced at the contact of CO2 with cast iron. If carbon monoxide (CO)
were formed, it would burn immediately to give CO2. Thus, to make things worse, we are considering unreal
conditions, since if the stove draws well it is because there is depression.
This means that if the cast iron is porous, there won't be any gas seeping
out. On the contrary, there will be an "air call" through the porous
wall!
But what really happened? Undeniably a red-hot stove in a
closed room brings about intoxication, sometimes deadly, from carbon monoxide.
The explanation of all this came to me indirectly when I was
fifty years old, although when I was still a child the problem had already
repeatedly presented itself. I had to wait until 1955 for a convergence of
mortal accidents to make me doubt the theory of the "invariance of
matter." Notwithstanding the current respect for official prescriptions,
there were nonetheless many people who had met death by carbon monoxide intoxication,
although it was attested to by many analyses that the victims could not have inhaled
carbon monoxide. I crossed the Rubicon, and with coworkers (eleven were
engineers from the finest schools of France) undertook a long series of
experiments. We secured the help of official laboratories along with the
cooperation-for blood analysis -of many M.D.'s. I abandoned my postulate
concerning the invariance of matter in order to confirm or nullify my
hypothesis about the real cause of death, intending to concentrate only on
results, whatever they might be.
In the meantime, in the spring of 1959 I was led to
conclusions, which revealed the explanation. Balance sheets had been established
on teams of workers. The Minister of the Sahara himself, Jacques Soustelle, an
ethnologist, had given me the opportunity to make a close study near petroleum
wells. In spite of the prevalent classicism, I decided in the summer to
publish these aberrant balance sheets established from chemical and physical
points of view, but resulting from research I had made with the cooperation of
the most eminent specialists of organic chemistry, together with professors of
medicine who believed that I was on solid ground.
As a high official of the French government, I had the
unique privilege of using any official laboratory. I could thus have the
collaboration of the most eminent specialized chiefs of laboratories,
professors of universities, etc. (for it is impossible nowadays to ask the same
laboratory to make different experiments). This privilege proved very useful to
me. Without such a synthesis of disciplines my task would have been impossible.
No isolated specialist could have succeeded. I thank all the eminent men of
science who brought me the support of their high qualifications, permitting me
to prove and confirm the validity of my theories.
On welders
In 1935 I made an observation, which left me perplexed. A
blowtorch welder was mortally intoxicated by carbon monoxide. My job was to investigate
the conditions surrounding the accident in order to determine the causes and
eventually prevent them. Nothing enabled me to discover the source of this
carbon monoxide.
Many times after that such accidents occurred, and on no occasion,
could I find the link, which would lead me to the origin of the inhaled carbon
monoxide. These facts remained in my subconscious until 1955. It was not until
that year that I saw the light. That same year in the space of a few months,
three oxyacetylene welders died. I received all the detailed reports,
including autopsies. All evidence indicated that the welders, who were all
steel cutters, had died from oxycarbonaemia and not from nitrogen oxides.
Analysis showed that the inhaled air had too small a percentage
of carbon monoxide to be dangerous. It was then decided, with the assistance of
M.D.'s working for the companies, to make blood analyses on all the victims'
comrades, although they looked in rather good health. We found that those doing
the same work were deeply affected with chronic oxycarbonaemia, some to a level
approaching that of fatal accident.
I put together all I had and imputed the fault to working
conditions, although analysis of the air inhaled by the workers proved that
there was no source of carbon monoxide anywhere. Investigations were made in
different places. The impregnation with oxycarbonaemia was general. After four
years of research, using the most delicate of methods, I could conclude:
1) that the strong blow-torches with which the workers cut
metal do not liberate carbon monoxide, but do bring a large surface of ferrous
metal to incandescence.
2) that those workers who were bending down to their work
and only they, not their helpers standing by – inhaled the air which licked the
ferrous and incandescent metal.
3) that the analysis of the inhaled air showed an absence of
carbon monoxide, meaning that the air was always a combination of nitrogen and
oxygen. This explained the fact that never, even though a great number of
investigations were made the world over, was carbon monoxide found in the
inhaled air.
4) that carbon monoxide – being detected in the blood of the
workers, not in the blood of the helpers – could only have an endogenous
formation when this air is breathed. In other words, it was activated by the
air's contact with a ferrous and incandescent metal. This would shed light on
the observations made in places using cast iron stoves heated to
incandescence.
The oxygen in the inhaled air was not sufficient to allow a
formation of CO in the organism. There is O, but C is needed. From where does
it come? After much research I thought that it was activated nitrogen, which
produced carbon in the organism (at the level of red corpuscles irrigating
pulmonary alveoli). This question hasn't been cleared up. The nitrogen
molecule (there is never free atomic nitrogen; isolated nitrogen is always in
the form of N2) contains two
nuclei of nitrogen enveloped by the electrons of the molecular orbit. In the
heart of the molecule the two nuclei vibrate at a known frequency. Were
this submitted to a vibratory and outer energy more or less of the same period,
could it be a resonance which at a certain time provokes the passage of a proton
with its neutron from one nucleus to another? All this occurs without any
change of the peripheral electrons. On one hand, there remains a nucleus with
one proton missing, thus carbon; the other nucleus acquires one more proton and
becomes oxygen. It is thus a phenomenon having nothing to do with nuclear physics;
one remains at the molecular state. But we have here an internal remodeling by
the removal of a proton from one nucleus to another. The measurements taken
show that in the molecule N2
the distance between the two nuclei of nitrogen is 1.12 Angstroms, whereas in
the CO molecule the distance between the C and O nuclei is 1.09 Angstroms.
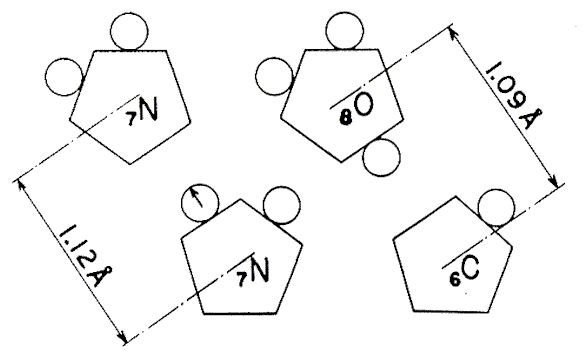
MOLECULE N2 MOLECULE CO
(PROTONS of the
ATOMS)
Fig. 1 The change of place of a proton – arrow on the left –
makes an N2 molecule become a
CO molecule.
This removal of a nuclei when a proton and its neutron exchange
change places does not seem radioactive: it could occur under the energetic
action of an unidentified enzyme at the level of the pulmonary air cells, or
perhaps in the thickness of the membrane of the erythrocytes going through the
air cell. It has not yet been proved that I was mistaken.
Controversies arose when I published the above explanation
in 1960. A specialist advanced the following
opinion: the heat of the inhaled air led to a dilation, a reduction of density,
thus to the rarefaction of the inhaled air.
From this would result a diminution of the oxygen pressure, which is
conducive to "bad combustion" in the blood, hence CO is formed
instead of CO2. It is easy to see that this hypothesis has
no value whatsoever. The breathing in of hot air would not lead to the same
effect if there were no contact with an incandescent metal.
Nevertheless, a systematic study* was made in 1963 by a
friend, Professor Desoille, and his colleague Truffert, both holding high
official positions. They demonstrated that the phenomenon was independent of
oxygen pressure. In 1964, I also showed that this phenomenon does not occur
when the metal sheet is brought to a temperature of 400º C; one needs at least
the deep red, and when the sheet is bright red the effect is quick. Oxygen is
not the cause; thus my first publications in 1960 were confirmed. Nor does this
same effect occur if one exchanges the nitrogen of the air with helium.
Nitrogen alone is the origin of this endogenous production of carbon monoxide.
The phenomenon being clarified, since my position in the scientific world
permitted it, I notified "inspectors" of the measures to be taken in
factories to avoid oxycarbonic intoxication once and for all.
[* H. Desoille, Absence de corrélation entre la pression de
l' oxygène et l' oxyde de Carbone dans le sang, Arch. Mal. Prof., July 1963.]
II POTASSIUM
It appeared useful to me to gather together all the results
of my research concerning the aberrant metabolism of potassium. This research
has permitted me to verify
1) that the vital phenomenon is not of a chemical order; it
goes deeply into the atom, starting in the nucleus. Organic chemistry is only
the final stage of molecular rearrangement.
2) that the nucleus of the atom in light elements is quite
different from what nuclear physics regards as the average type, the latter
having value only for the heavy elements.
3) that Nature moves particles from one nucleus to another particles
such as hydrogen and oxygen nuclei and, in some cases, the nuclei of carbon and
lithium. There is thus a transmutation.
4) that biological transmutation is a phenomenon completely
different from the atomic fissions or fusions of physics; that it reveals a
property of matter not yet seen prior to this work.
My research was directed mostly towards the reactions taking
place inside the nuclei of sodium, magnesium, potassium, calcium, nitrogen, and
in a lesser degree, phosphorus, sulfur and chlorine, etc. The major role of
potassium appeared to me to be a biological regulator; it can be produced
endogenously from sodium. This reaction allowed me to calculate the endothermal
energy necessary to tie a nucleus of oxygen to a nucleus of sodium, giving a
nucleus of potassium:
This process necessitates only one millionth the energy of a
reaction of nuclear physics in vitro.
Relation between potassium and temperature
There is abundant literature concerning this subject. Here
are a few experiments cited by Reinberg:
It seems that it was Bachrach who first studied this phenomenon:
he made cultures of lactic bacteria and brewer's yeast in a hyperpotassic medium
at different temperatures. After one month the multiplication of these
unicellular organisms was maximum in media richer in potassium, at the highest
temperature. (With high temperature but a small amount of potassium the results
are different.)
Ets and Boyd (study on the sciatic nerve of the frog) showed
that cold is inhibitory. When potassium is added, this blocking occurs – but
only at higher temperatures; the effect is even more accentuated when the K
rate is higher.
Hundreds of experiments of this sort have revealed a
correlation between the temperature at which the metabolism of living tissue
occurs and the tissue's potassium content. But the nature and cause of this
relation have not been determined.
E. G. Martin verified that an excess of potassium stops the
heart of a fresh-water turtle cultured in a cold medium. If the temperature is
raised, the potassic inhibition ceases. There is then a maximum potassium
concentration not to be exceeded and a minimum, which must remain in proportion
to the temperature. Let us note that, for man, the "fork" in the
plasma is from 150 to 200 mg/l, variable with every individual, but beyond 300
mg/l there is danger. If a relation between K and temperature has been seen, it
does not seem to have perceived that this is a relation of opposition: an
excessively high temperature slows or stops physiological activity; the
organism reacts by secreting K.
Relation between potassium, oxygen and hydrogen
The relation between potassium, oxygen and hydrogen has been
perceived and various works contain references to it, but here, too, no general
explanation has been seen.
a) Relation between potassium and oxygen
It has become apparent that potassium is most abundant where
oxygen is present, i.e. where the metabolism is very active and the breathing
deep – thus, in fully active tissue.
It is necessary to keep in mind the reaction Na + O = K, in
order to understand that endogenous potassium is possible only if it has access
to oxygen for its formation. This fact sheds light on the following findings:
Latshaw and Miller established that in corn, on the average,
45% of the plant's total potassium is in the leaf (where respiration is
strong), 35% in the stem, 13% in the kernel, 4% in the bale, and 3% in the
root. These values vary, of course, according to the age of the plant, to
season, and also to light (which is not surprising, since respiration is
linked to chlorophylian photosynthesis). It has been verified that in potato
leaves K is at its maximum during the day and at its minimum at night.
Broyer showed that a small amount of oxygen increases the
potassium content in the roots of barley, tomatoes and rice. The same is true
of man and animals, where the potassium content is directly proportional to
breathing activity or to the activity of tissues, which require much oxygen.
That is why cancerous tumors are richer in K, a fact having
been verified in man and in the sarcoma of the chicken (Moravek).
An increase in K content leads to an elevation of arterial
tension, to the activation of the vasomotor reactions. (The opposite is true
with Mg and Ca). An increase of K from an injection in the cerebro-spinal
liquid provides an intense breathing stimulation.
An animal is put to sleep with an injection of Mg and awakened
with K (by an injection in the infundibulary area, with no effect in other
areas of the brain). Oxygenation slows down during sleep or under the influence
of narcotics. Potassium does the same. During sleep the metabolism is slowed
down. There are less exchanges, less O, and thus less K; this reduction of K
at the end of the sleeping period can attain to 16.6% in the plasma.
An excess of K diminishes the frequency and amplitude of the
systols; at the extreme there is heart failure, the muscles and arteries having
been loosened. Muscular activity necessitates oxygen, thus leading to an
increase of K in the extra-cellular medium. Tipton verified this on the cat,
Heppel on the rabbit, Bureau on the frog, Fenn on man, etc.
Heart failure in man occurs when K = 9 to 12 m Eq/1 in the
plasma (approximately 350 to 450 mg/1).
For more details concerning the effect of K on the heart I
refer the reader to specialized books. It follows that there is a Na/K
relation, which must remain within limits, and that this required balance also
implies limits for the K/Ca relation. One sometimes studies the Na + K / Ca +
Mg relation. Darrow showed that the K variation greatly affects the
electrocardiogram, which reveals a hyper or hypo "potassemia" or
"kalemia"; this is due to a lack of polarization which is itself
dependent upon the relation between Na and K on each face of the superficial
layer of the nervous fiber. The ratios of concentration between Na in the
outer medium and K in the nervous cell modify the difference in potential.
A young rat needs 15 mg of K per day. It needs only 2 mg
when it is an adult. The average amount of K necessary for a human baby is 9
grams per day, which means that a nursing mother's milk, is relatively rich in
K (500 mg/l) but poor in Na.
b) Relation between potassium and hydrogen
If there is abundant literature showing that the presence of
K is dependent on the availability of oxygen, there are also several
experiments showing its relation to hydrogen, for according to our reaction, K
+ H = Ca. In other words, if K is too abundant in the presence of H, it will
give Ca.
The presence of H is linked to acidity (low pH). An excess
of H ions signifies an acidity that might become dangerous for the cell.
However, in that case K can join an H nucleus to produce Ca,
thereby establishing alkalinity and an optimum Ca/K ratio.
The agent of equilibrium is thus K. The effects between K and Ca are opposite
in appearance only; they are in fact complementary.
Hoagland writes that there is a clear tendency toward acidification
of the cellular medium, freezing H+
ions; the addition of K+ ions
leads to the alkalinization of the cellular liquids.
Reinberg notices that "the alkalinization of the
cellular liquids with K is well known by arboriculturists, who use potassium nitrate
to speed up fruit maturation."
It is of interest to point out that the proportions of K and
Ca are of the same order in animal life – in the plasma as well as in seawater,
where life began. Here is a Reinberg table:
Contents
in m Eq/l
K Na Ca Mg Cl
Sea water 10 450 20 100 530
Rat 6.2 145 6.2 3.2 116
Dog 4.7 142 4.9 1.8 108
Man 4.5 140 5 2 102
If one compares the weights in milligrams, one finds that
Na/K in seawater is from 25 to 27, in the plasma of man from 17 to 18 (varying
from 15 to 22 according to the individual), but in cells it is K, which
predominates. In the red globule, the vehicle of oxygen, Na gives an abundance
of K. Na + O = K, because K/Na =
approximately 180!
In sea animals the percentage is almost the same as in
seawater. In fresh-water or land animals it is lower, but there the K/N a ratio
is much higher. This indicates a more active life and more oxygenated blood,
since K and O "go hand in hand," while Na decreases.
However, in land animals we find that the K/Ca ratio in the
plasma is very close to 1, due to the reversibility of the reaction
K39 + H1
<=> Ca40
Conditions are different inside the cell; it is here that
the reaction takes place. Na penetrates the cell and fuses with O to give K;
thus there is less Na and more K, but little Ca.
The following is another of Reinberg's charts: (m Eq/l)
K
Na Ca Mg Cl
Octopus' muscle 101 81 3.7 12.7 93
Cat's muscle 151
28.5 1.2 15.4 18
Man's red blood corpuscle 105
10 0 5.5 80
The cells of land plants are richer in potassium and Ca and
poorer in sodium than those of animals. A few examples:
K Na Mg Ca
Mushroom 102 8.7 4 11.5
Potato 115 8.7 25 7.5
Chestnut 135 8.7 33 20
Wheat (grain) 118 8.7 112 22.5
Corn (germ) 197 36 440 34
Date 165 4.3 52 32.5
Asparagus 64 1.3 9.1 12.5
Strawberry 40 2.2 17.5 22.5
Grape 64 2.7 8.3 5
Darrow pointed out that a K increase in the cell decreases
the cell's acidity because it causes a decrease in H. Thus the alkalinization
takes place when K takes H to give Ca.
Ca is taken back by the outside liquid and excreted, producing a
negative Ca balance sheet. More Ca is excreted than ingested, but the main
source of Ca is Mg:
The internal equilibrium of the animal cell postulates a
large K content and a small Ca content. The reactions with H help to reduce
acidification, since H is taken away.
It has been found that micro-organisms in the soil excrete H
ions which acidify the soil; however, K neutralizes this acidity when it comes
in contact with the roots.
If the calcium concentration in the nutritive medium is increased,
there is a smaller absorption of K. This can be explained by the fact of
reversibility:
K + H <=> Ca
This specific reaction allows a biological equilibrium to be
maintained.
We shall learn more about the metabolism of potassium in
other chapters. This present chapter was intended to acquaint the reader with
some simple facts as a basis for helping him understand what the book hopes to
convey.
III SODIUM –
POTASSIUM
We have seen how sodium can be transmuted into potassium.
This reaction is very important in animal biology. I have described it many
times in my earlier books.
I confirmed this phenomenon, thanks to the ethnologist
Jacques Soustelle, Minister of the Sahara, who gave me the opportunity to make
experiments on teams of workers. The latter were busy drilling wells under
extremely difficult conditions. It is common knowledge that in the Sahara
Desert it is dangerous to remain too long in the sun. The fact that people
could work hard on metallic platforms unshaded from the hot summer sun remained
unexplained. Systematic research was conducted with the help of a military
doctor and his assistants.
A voluntary team was followed carefully for six months. Everything
they ingested and excreted was weighed and reported. The balance sheet showed
that during great heat the potassium emitted through perspiration was greatly
increased. However, sea salt ingestion increased also. The workers were given
extra salt in the form of tablets, which they sucked. But this ingested salt
was not entirely secreted. What happened to it? It could not possibly have been
stored in the body, because the difference between ingestion and excretion was
so great that an accumulation of it would have been impossible.
The biggest mystery lay in the thermal balance sheet. By
their work and food, and by the heat endured in the sun and in the shade
(ambient temperature was higher than body temperature), the workers averaged
4,085 kilocalories per day in those six months, reaching more than 7,000
kilocalories per day in the summer. Perspiration averaged 4.12 liters per day,
and due to the extreme dry heat it did not even drip, but evaporated
instantly. 540 kilocalories are needed
to evaporate a liter of water. With such an imbalance the workers should have
died from "hyperthermia" because the heat could be released only
through perspiration – that is, 540 X 4.12 = 2,225 kilocalories, and 4,085 –
2,225 = 1.860 kilocalories per day, according to the classical balance sheet.
Such an excess is obviously impossible.
I came to the conclusion that it was sodium which, disappearing
to become potassium, created an endothermal reaction (thus causing heat to be
absorbed.) Hence by instinct one consumes more salt in a dry and hot country.
This is why salt is so important in Africa, the Middle East, etc., where
caravans travel up to 1,000 kilometers to bring back salt. In Taoudeni, a
unique city in the middle of the Sahara 1,000 kilometers north of Timbuktu, the
monetary unit is the salt bar. Also, notice the importance given salt in the
Bible.
The transmutation from sodium to potassium was confirmed by
another experiment made in a more arid part of the Sahara with the help of the
Marine Militaire. This experiment took eight months. Systematic research was
carried out in a physiology laboratory where it was found that a man making a
major physical effort during three hours, in a temperature of 39° C (102.2° F)
with a humidity of 60%, would experience an increase of three times his usual
rate of potassium in proportion to sodium, in his urine. This reaction has a
biological significance. It has been commonly known that people struck with a
lesion in the surrenal glands reject much more potassium, even if it is not
given to them. It was never understood from where this potassium came – the
small reserve, which the organism can mobilize, does not justify such a massive
excretion! (On the other hand, salt has been found to disappear in the
organisms of people inflicted with Addison's disease.)
Blood plasma is very rich in sodium chloride (sea salt), containing
approximately 7 grams per liter. However, the rate of sodium chloride
diminished in the blood even with normally salted food. This enigma was classified
and forgotten among the mysterious phenomena of life, the sodium-potassium
relationship ignored.
M.D.'s have seen the blood's potassium increase at a
dangerous rate. Excess potassium diminishes nerve excitability, making the
electric potential equal in the two faces of the nervous cell wall.
Normally the outer medium of the cell is richer in sodium
and poorer in potassium than the interior of the cell. The ratio between the
ions of potassium in the interior and exterior of the cell defines the membrane's
potential. Abnormal balance results in a paralysis of the nerves of the heart
and lungs; this in turn causes syncope and ultimately death. Some doctors
thought that by replacing plasma too rich in potassium with an artificial serum
containing only sodium chloride, they would achieve good results Unfortunately
this attempt was followed by the immediate death of the patient.
The reader has probably discerned that the potassium came
from sodium and that whenever fresh sodium is injected into the organism, it is
immediately transmuted into potassium.
Professor Perrault, a "famous hospital boss" and a
member of the Faculté de Medecine of Paris, once asked me to give his students
a lecture explaining what was really happening. It had been found that aldosterone
had provoked this transmutation. In cases of surrenal lesion the opposite
hormone is not sufficiently secreted and the balance is lost.
Reactions of this kind occur in accordance with the
physiological condition of the patient. Thermal balance sheets of food
calculated by dietitians have a relative value; according to chemical
experiments made in laboratories, chemical energy is released only by the
combustion of carbon in food, most of all in carbohydrates (sugar).
The sodium-potassium link presents itself in many varied
forms A study made on terrestrial and marine iguana showed that some species
secrete from a special nasal gland a liquid containing up to 190 times more
potassium than is in their blood plasma, at a rate of 190 cm3 per hour. A solution
of sodium chloride added to the cesspool of these reptiles stimulated a
potassium increase in the excretion of their nasal glands, but no sodium
increase. If potassium chloride has been added, the potassium concentration and
the glandular flow would still have increased.
Dr. Jullien* (from the Faculté des Sciences of Besançon) has
proved that if tenches are put in water salted with 14%0 sodium chloride, the
rate of potassium chloride rises from 3.95 g/l to 5.40 g/l after four hours,
i.e. a 36% increase.
[*Annales
Scientifiques de l' Université de Besançon, 2" Śerie Zoologie et
Physiologie fasc. 13-1959]
The same result can be achieved in three days (72 hours)
using water salted with 8% NaCl. KCl
passes from 3.95 g/l to 5.39 g/l. The
calcium chloride content remains 0.300 g/l from beginning to end in the
experiment. In order to be sure that it is not the cellular potassium of the
blood globules that enters into the plasma, one must take the precaution of
measuring the total K in the blood (plasma + globules).
This potassium increase cannot be attributed to a loss of
water, for the relative concentration of all the salts would then be uniform.
We have seen that the concentration of calcium salt does not change; there is absorption
of NaCl only, hence a slight sodium chloride increase in the blood. The NaCl
content increases from 5.10 g/l to 6.60 g/l after four hours in water salted to
14%0, and to 6.40 g/l in 72 hours in water salted at 8%0, which is a 25;;
increase as opposed to 36% for potassium chloride (with no variation in the
calcium chloride content) .
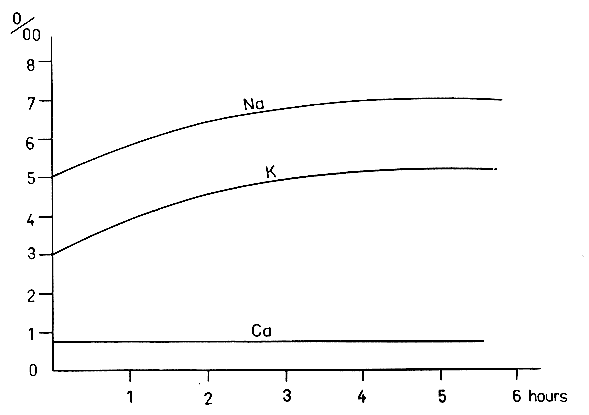
Fig. 2 Variation of
Na, K, and Ca in the blood of a tench in water containing 14% NaCl.
The problem of the passage from sodium to potassium is of
great importance in physiology. This valuable mechanism of nature insures the
thermal regulation of the organism. The reader will recall the experiment made
in the Sahara, in which case the variation of the K/Na balance sheet was
remarkably parallel to that of the thermal balance sheet.
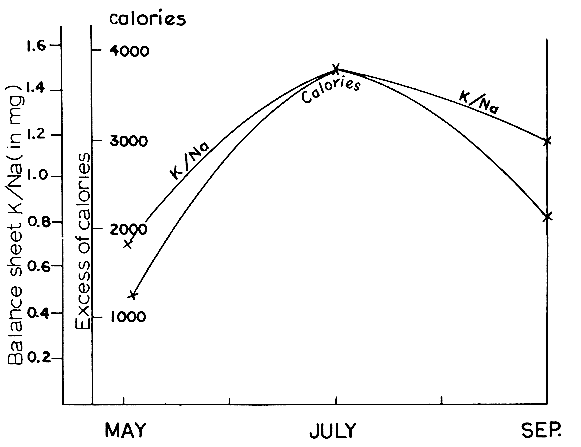
Fig. 3 K/Na and thermal balance sheets.
The body was receiving more heat than it received while it
temperature remained normal, due to evaporation, perspiration etc. In
physiology laboratories, experiments made on men have shown an increase in
potassium excretion under hot conditions if the organism can dispose of sodium.
(This observation justifies such "empirical" practices as giving hot,
salty vegetable broth in cases of fever.)
An addition of potassium allows a tissue in culture to
continue living at a higher temperature. The secretion of potassium is thus a
defense reaction by the organism, occurring in cases of accidental increase in
temperature. It establishes a new equilibrium. (For example, the fever remains
constant at 39° C. {102.2° F.}) It
appeared that here was the explanation of how an organism combats fever: the
transformation from sodium to potassium is made through a strongly endothermal
nuclido-biological reaction (sodium + oxygen). From the K/Na and thermal balance
sheets, which I had made in the Sahara on two similar teams, I obtained a
quantitative comparison indicating the value of the endothermal energy
resulting from this reaction. Let us recall the practical applications, such
as salty drinks for the prevention of hyperthermia among workers being exposed
to dry heat. M.D.'s and professors of medicine are now better able to
understand the mechanisms of fever; for it is obvious that the thermal
equilibrium's being at an unusually high temperature does not result from the
perspiration evaporating. Thus the specific heat of the evaporated water does
not affect heat loss, whereas the inflammation, which is the cause of
hyperthermia, continues to supply calories. It is an endogenous and endothermal
reaction, which maintains the equilibrium by an intense excretion of potassium,
showing that the potassium is produced in the organism, which then rejects the
excess.
Much research in biology has been done concerning oxygen
consumption in cases where sodium increases and potassium decreases. "The
oxygen consumption of some invertebrates (as seen in the snail's heart, mussel,
etc.) increases, depending on the Na/K ratio," writes Reinberg in Sodium
and Life. If there is a shortage of oxygen, there is no longer a diminution of
Na accompanying an increase in K. Na and O are thus necessary for verifying
the increase in K.
In Annals of the New York Academy of Sciences (July 1966), a
collective volume of more than 600 pages, dedicated to the recent progress in
the study of biological membranes, we find under the signature of H. H.
Ussings: "The excess oxygen consumption seems to derive from some anomaly
in the handling of sodium" (p. 544); or, "It is seen that in all
experiments the oxygen consumption per equivalent ion (of sodium) is much
higher than normal" (p. 545); and, "The oxygen consumption is
increased in proportion to the amount of sodium transported" (p. 553). The
author verifies these facts without attempting to explain them.
The "transport" of sodium is generally considered
to be an exchange with potassium through the cellular wall – potassium being
more abundant in the intra-cellular medium than in the exterior one. This is a
classical theory. There is no reason to doubt it; the number of experiments and
their variety provide confirmation of this phenomenon. But one is almost
always satisfied with such an explanation – an explanation mistakenly
generalized, which might be true qualitatively but not quantitatively.
There is no simple exchange through the wall. Liechtenstein
and Leaf (1966) recognize this fact. They say, "However, previous studies
have demonstrated no quantitative relationship between net sodium transport
and potassium uptake from the serosal sodium," and, faced with the
contradictions of classical hypotheses in opposition with the facts, they add,
"Further studies, in fact, have led us to the somewhat uncomfortable
conclusion that the major effect of removing potassium from the serosal medium
is to somehow reduce the mucosal barrier's permeability to sodium, so that
insufficient sodium can gain access through that surface."*
[*Annals of the New York Academy of Sciences, July 1966.]
The authors came to another uncomfortable conclusion. They
saw a possible explanation from the classical point of view: that there is no
proportionality between the potassium extracted from the serosal medium and the
sodium that is removed. Thus it is not a case of exchange, as has always been
maintained by the orthodox, for we have seen that it is impossible to
postulate a one direction movement of potassium without stipulating a
disappearance of sodium to achieve a quantitative equilibrium of matter and
electric charges.
This observation enables us to understand why a great specialist
of hormonal problems, Perrault, professor at the Faculty of Medicine and chief
of a department of a large hospital in Paris, could have verified long ago that
"potassium was coming from nowhere." Without being supplied,
potassium appears in great quantities; it can only have been created on the
spot. In 1963 he introduced the only
explanation possible in view of these verifications (in the cell): (1) no
introduction of Na; (2) diminution of
Na; (3) increase of K; (4) oxygen consumption. In other words, it
could only be the reaction producing the biological transmutation that we have
described: 11Na
+ 8O
:=: 19K. Three years later many research workers all
over the world were obliged to recognize that the exchange through the walls is
a partial view only, insufficient quantitatively speaking, and that the four experimental
facts mentioned above are indissolubly bound and occurring simultaneously. Even
before Perrault made his observations, at least three Sorbonne professors, to
my knowledge, had introduced this phenomenon in their teachings. Since 1963
many more university professors have joined them, as I learned by accident. I
have also the names of a few professors in colleges and schools of agriculture,
engineers and agronomists who have presented these notions in class teachings,
newspapers, or lectures. There is, to my knowledge, at least one brochure
edited by a group of teachers explaining a few of the biological transmutations
of the elements, for the purpose of enlightening primary school teachers. An
agricultural correspondence school devotes a whole chapter to it.
I should never finish if I were to cite all the observations
that have been made concerning the relation between Na and K. In Reinberg's
book* alone there are pages about it. For example: "The optimum
temperature, corresponding to the maximum work of the heart, is proportionally
lower when the value of the Na/K relation in the medium is higher. There is a
contractile inhibition in the sodic medium; one increases it by adding K."
[*Potassium et la Vie, P.U.F. Pub., 1955.]
In a culture in vitro one should add potassium, for an
isolated tissue cannot produce it; the aldosterone, which is secreted by the
cortex of the surrenal (according to a mechanism by which the hypophysis
intervenes) is the main hormone performing this reaction. "An excessive
supply of sodium can determine the increase of the urinary elimination of
potassium" (Reinberg).
The studies are many and all converge in one direction.
Watan has shown that kidneys continue to secrete potassium even when a special
diet deficient in potassium is followed for several weeks. Lehman (Director of
the physiology laboratory of Dortmund) declares, "The excretion of
potassium does not make us appreciate its absorption." He also writes,
"The increase in potassium excretion during work at high temperatures is
not due to a larger supply of potassium."
It must be made clear that experiments and discoveries of
this kind show that in abnormal conditions the organism proceeds with an
accelerated transmutation from sodium to potassium.
One should not then conclude that potassium is not useful
under normal conditions, since sodium can produce it. The sodium-potassium
relation should be put into perspective in order to answer certain questions
asked by dietitians.
IV CALCIUM
This chapter won't involve a detailed study of the verified
abnormalities in calcium metabolism. It will simply attempt to attract
attention to the origins of calcium in order to show how the reactions that I
have established modify present views in fields other than biology.
Calcium is one of the most abundant elements in the earth's
crust (3.25 %). Oxygen comes first
(49.13 %), then Si (26.0%), Al (17.45%), and Fe (4.2%).
If the great formations of limestone are from the Secondary
Era, how is it that one nevertheless finds them before the Primary Era, in the
Pre-Cambrian? They are being formed nowadays in animals and plants, and we see
that calcium has three origins:
– It can come from potassium:
K39 + H1
=> Ca40
– from magnesium:
Mg24 + O16
=> Ca40
– from silicon:
Si28 + C12
=> Ca40
These three potential origins of calcium are by far the most
important; from them alone I have gleaned valuable observations and
experiments.
Does this mean that there are no other possible origins? I
would not risk making such an assertion. Let me say only that I have grounds on
which to base such research. What I can say for time being is that these other
origins are quantitatively of little importance.
Isotopes of calcium
I would like to point out that heavy hydrogen (2H) or deuterium (D) rarely enters into
these nuclido-biological reactions, nor have I ever found such to be the case
throughout my research. I will neglect, then, the deuterium reactions, since I
have never been able to bring them to light. This does not mean that they do
not exist; one could perhaps find transmutations made with 2H. However, they are rare and of small
quantitative importance. Here are the reactions that I have verified in my
research concerning the origin of calcium:
a) Potassium as a base:
K39 + H1
=> Ca40
K41 + H1
=> Ca42
b) Magnesium as a base, with stable isotopes of oxygen:
Mg24 + O16 => Ca40
Mg26 + O16 => Ca42
Mg25 + O17 => Ca42
Mg25 + O18 => Ca43
Mg24 + O18 => Ca42
Mg26 + O18 => Ca44
c) From stable isotopes of silicon and carbon:
Si28 + C12 => Ca40
Si30 + C12 => Ca42
Si29 + C13 => Ca42
Si30 + C13 => Ca43
Thus Ca40,
Ca42, and Ca43 can come from K, Mg, or Si; but Ca44 can only come from Mg.
One must then be prepared to admit that the calcium formed
by shells and originating from the magnesium of seawater is richer in Ca44, more so than if the shells' formation
had taken place on land. Organisms succeed in doing the transmutations better
with heavy isotopes, making a greater proportion of heavy isotopes necessary in
elements of organic origin. (This proportion is extremely variable, so much so
that the proportion indicated in the tables of nuclear physics can be only approximate.)
Thus one should avoid using the numbers given in the Periodic Table of
Elements, where one is presented with raw forms of mineral elements, which are
mixings of isotopes having no value in biology. The given figures of these
tables are used in chemistry, but are too gross to be used in the study of the
nuclido-biological reactions where nature operates at the level of the
nucleus. (Chemistry deals with the molecular level.)
As an example, let us take the reaction Si + C => Ca. The tables give:
14Si + 6C
28.06 + 12.01 =
40.07
But these same tables give 40.08 as the atomic mass for Ca,
not 40.07. There is indicated a mass gain, thus an emission of energy, if these
inaccurate figures are referred to.
The following is an example proving that one should not use
the Table of Elements' atomic mass numbers in studying the nuclido-biological
reactions. As a matter of fact, the same applies to calcium, whose origin is
either potassium or magnesium.
K + H =>
Ca
39.096 + 1.008 =
40.104 (false figures for Ca =
40.08)
Mg + O => Ca
24.32 + 16 = 40.32 (false figures for Ca = 40.08)
There is always a mass gain indicated, which is false.
We have seen that a priori, according to the laws
that we have deduced from experiments, there should be more Ca44 in shells and in other animal and
vegetal organisms that make their calcium from the magnesium of seawater. This
has been verified: the more active the organism, the more oxygen it consumes
and the richer it will be in Ca44. An organism's activity is proportional to
the existing temperature: its metabolism is more active in warm media than in
cold. It follows that a shell will have a higher Ca44/Ca42
ratio if the animal, which secreted it, is from a warm sea. A study of this Ca44/Ca42
ratio in fossil shells has been proposed, to determine the temperature of the
sea during the epoch corresponding to the fossil's life.
Calcium production by plants
Von Herzeele established around 1850 that germinating seeds
without a supply of calcium saw a calcium increase in the young plants analyzed
30 days after the germinating process.
The results were contested because they were contrary to
Lavoisier's law. However, the operatory precision von Herzeele employed left no
room for doubt. P. Baranger, Chief of
the Laboratory of Organic Chemistry at l'Ecole Polytechnique de Paris thought
that von Herzeele's analyses were insufficiently precise, and had the curiosity
to conduct the experiment all over again with all modern scientific rigor.
The results of his analysis executed on three equal portions
of 200 identical seeds, each one weighing 7.2 gr, were submitted to a
professional statistician in order to detect any possible operatory error.
Taking only the most typical case, where the seed sprouts had only a supply of
doubly-distilled water (water distilled only once is not pure enough), and with
all chances of systematic error having been averted, there was more calcium
found after the germination.
Thus scientific proof that calcium can be created in
biological reactions has been acquired in one of our most celebrated schools.
But no interpretation of the phenomenon was offered.
V POTASSIUM –
CALCIUM
Reversible Transmutations
My youthful observations gave birth to a subsequent idea of
a possible transmutation from potassium to calcium. Hens in a granitic region
devoid of limestone can lay eggs with calcareous shells every day; but these
hens, free to move around in the yard, scratch about for fragments of mica
strewn on the ground.
In a clayey area hens need limestone, but not if mica is
present. The difference between clay and mica is that the latter contains some
silicate of potassium.
1) Hens kept in a chicken coop on clayey soil were without a
source of limestone. After a few days their reserves were exhausted and the
deficiency became apparent as eggs with soft shells began to be produced. On
that same day, purified mica was given to them. The hens jumped on it and began
scratching around it very rapidly, panting over it; then they rested, rolling
their heads on it, threw it into the air, and began scratching it again. The
next day eggs with normal shells (weight 7 gr) were laid.
Thus, in the 20 hours that intervened, the hens transformed
a supply of potassium into calcium. Two eggs are never produced at the same
time; one egg is laid and the shell of the next one begins to be formed a few
hours later. The egg composition is determined by the food eaten the week prior
to the laying of the egg. But the shell, on the contrary, is formed by a rapid
excretion. An experiment of this kind, using the same mica, was undertaken
with guinea-fowls over a period of forty days. The administering of the mica
was suspended three times and each time a soft-shelled egg was laid, providing
new evidence to complement the numerous observations which have shown that
potassium cannot be stored in the organism.
2) One of the observations that intrigued me and contributed
to my "conversion" was the presence of saltpeter in the limestone on
walls. This phenomenon is not new to man. Saltpeter was used in making
gunpowder before man learned, in the last quarter of the nineteenth century,
how to make saltpeter (which is potassium nitrate) from potassium chloride.
Saltpeter has been ex traded not only from limestone on
walls, but from soil of calcareous regions having a humid, warm climate
alternating with a dry season. Right at the beginning of the dry season the
soil is covered by a thick white layer resembling snow. The first gathering of
saltpeter can be followed by a second one only a short time later.
On the walls of my house at the seashore I noticed that saltpeter
sprang up continually, despite my frequent efforts at scraping it. It is doubtful
that the limestone contained such quantities of potassium, for I had removed
the saltpeter many times a year for eleven years.
I thought then that only calcium could be the origin of
potassium ( calcium – hydrogen = potassium).
2040Ca – 11H := 1939K
I compared this experiment with the one in which hens made
eggs with calcareous shells without the use of limestone, after they had
consumed mica. (Mica contains potassium silicate.) In the hens the opposite
reaction occurs: potassium + hydrogen = calcium.
Thus there is a reversible reaction in nature. The parallels
between these two reactions helped lead me to the hypothesis that
transmutation occurs with an addition or subtraction of hydrogen, by the
removal of a proton only, at the level of the nuclei.
This experiment on hens proved that there is a calcium–potassium
relation, with potassium becoming calcium. This reaction is opposite the one
that produces saltpeter. A transformation from potassium to calcium is also
verified in man, in whom it aids calcification (but only in a very small
degree).
Here is a nuclido-biological reaction with + H (which means
that it is reversible, but not in the same organism):
1939K + 11H :=:
2040Ca
Passage from
calcium to potassium
In a report made on the formation of granite G. Choubert
cites an aberrant observation. Limestone of the second Pre-Cambrian Age can
give potassic adinolites containing up to 12% K20. The author writes, "The
limestone is first transformed into marble. Then suddenly, without undergoing
any transition, it changes into adinolite. What process other than some
mysterious one could explain a transformation from limestone to a silico–potassic
rock at the contact of a calco-magnesian magma?"* (These caleo-magnesian rocks are the
dolerites, gabbros, and pyroxenolites. )
[* "L'origine des granites et la physique
nucléaire," published in the bulletin Notes du Service Géologique, Vol.
VI, Rabat 1952.]
A study of certain adinolites has shown the latter to have
been formed from a contact with dykes and dolerites incapable of producing
such quantities of potassium. Mr. Choubert writes, "The potassic input
could have come from nowhere ... hence potash is born on the spot by atomic
reaction."
We can thus see how in 1952 Mr. Choubert had perceived the
situation clearly. An explanation of this phenomenon was afforded by our
experiments, which showed that transmutation did occur on the specific element
cited by Choubert.
VI PRODUCTION
OF CALCIUM FROM SILICON
For those who doubt the reaction Si + C => Ca, showing that at the nuclear level
calcium can come from silicon, it will be enough to point out a few
observations made by different scientists.
After I had discovered the reaction Dr. Charruyer, Chairman
of the Department of Physics at the Medical School of Limoges, mentioned to me
that he had found in primitive grounds geodes of calcite in slaty rocks which
were very hard, compact, and absolutely impermeable. These rhomboidal forms of
calcium carbonate can be very big and weigh many kilos, but due to their
impermeability, there is no possibility that they could have come by migration.
They could only have had an endogenous origin in one of the components of the
schists. In my opinion they could only have come from the reaction given above,
since C also comes from the schists in the reaction Si => C + O.
The ability of silica to change into limestone has been
recognized for ages, since in antiquity horsetail (Equisetum) was used for
recalcification. (Horsetail is rich in silica.) It was also used for curing
tuberculosis because it speeds up calcification of the lung caverns, thus
promoting quicker healing. In 1846 Pierre Jousset, one of the great masters of
homeopathy, showed in a thesis the effect of silica on people stricken with
tuberculosis.
P. Van Thieghem had pointed out in Traité de Botanique
(1899) that in the thallus of fucus, which grows in siliceous rocks, there is a
high proportion of calcium sulfate.
This production of limestone by organic silica, as explained
by the reaction Si28 + C12
=> Ca40, has only recently attracted the attention of
modern scientists ... even though the phenomenon was generally known in
antiquity.
The therapeutic method of recalcification has been used only
rarely, for the most part by doctors who are traditionalists, healers, and
homeopaths, etc., believing in natural remedies.
Wasn't it heresy to say that silica could recalcify? It was
a denial of Lavoisier's chemistry, still official. That is probably why plants
rich in silica, such as horsetail, were very recently erased from the
pharmacopoeia in France.
In fact it is not unusual to meet people who still use
horsetail in decoction or infusion. Some laboratories make an extract of it. It
has been put on sale in pharmacies, but in order that the label conform with
the pharmacopoeia, tricalcic phosphate is added to it.
Another horsetail extract, prepared differently by another
laboratory, does not contain added calcium; it is presented as a dietetic
product for all those wishing to avoid the risk of decalcification. Many
pregnant women use it.
Nature has many ways to prevent a deficiency resulting not
from the lack of an element but from an insufficient production of the enzyme
that carries out the transmutation. Calcium is not assimilable, at least not
for man-unless it is proved otherwise. Decalcification may occur when there is
a deficiency of the enzyme, which transmutes sodium into magnesium, but it
usually occurs because of a deficiency of the enzyme, which transmutes
magnesium into calcium. It is much better to recalcify with potassium and
organic silica.
Decalcification can then occur when saltless diets are prescribed,
especially diets without chloride.
There has never been a counter-indication for the use of
silica, except in excess as with any kind of food; however, the body's silica
tolerance is great.
Organic silica's action is fast: nails cease breaking and
become normal after fifteen days if extracts are used; a longer time is
required for horsetail decoctions.
Spectacular results have been obtained in the repairing of
broken bones, but this we shall see in a chapter specifically dedicated to
medicine. I shall demonstrate that fractures are repaired and healed faster
with organic silica extracts than with the administration of calcium. Mineral
calcium is a residue and the organism does not assimilate it; it is found in
this terminal stage in man and in higher animals. However, plants have the
opposite reaction and can use calcium directly.
For man, organic silica (which can only be found in plants
in the springtime) must be used, because mineral silica has the opposite
effect: it decalcifies.
* * * * *
The relation of silicon to calcium is also apparent in a
careful study made on an egg in incubation. A chicken just hatched has a
skeleton of bones and thus of calcium. However, there is an insufficient
amount of calcium in the egg. Nevertheless, at the time of birth the chick's
skeleton contains four times more calcium than the egg (both yoke and white).
It has been debated that the calcium comes from the shell again
a groundless assertion. Many research workers intrigued by the disproportion
between the calcium in the skeleton and that of the egg wanted to see if there
really was a migration from the shell. However, the latter has never been
proved.
One can point out that the composition of batrachians and
fish eggs is approximately the same as that of birds, and they have no
noticeable calcareous envelopes. At its hatching the little one nevertheless
has a calcareous skeleton, even in a fresh-water area lacking in calcium.
Research workers have discovered that the calcium weight in
the egg does not change up to the 10th day. At the moment the membrane under
the shell breaks away from it, the air chamber grows bigger – there is no
possibility of migration of calcium toward the egg.
This membrane under the shell contains organic silica – approximately
0.5% in the outer leaf – in proportion to the fresh matter weight.
Limestone increases in the egg at an average of 0.04 g on
the 10th day to 0.05 g the 14th day and 0.06 g the 16th day; then ossification
comes about suddenly and the limestone attains 0.10 g on the 17th day, 0.13 on
the 18th day, 0.17 on the 19th day, and 0.18 on the 20th. Thus from the 16th to
the 20th day the limestone triples.
Most recent research shows that limestone "grows"
when it comes in contact with the outer leaf of the shell. Dr. A. Charnot,
Chief of the laboratory in Rabat, has verified that the membrane under the
shell contains, for 100 g:
154.79 mg of silica (SiO2) in the inner leaf
464.80 mg of silica in the outer
leaf.
For further information on the production of calcium from
silicon, see the chapter on medicine and nutrition.
VII MAGNESIUM
Endogenous
Production
The link between "dolomie" and limestone is well
known, although it has never been explained. It has been verified and that is
all! In their uncertainty, specialists use terms such as "consanguinity of
calcium carbonate and magnesia," "genetic consanguinity,"
"unitarian genesis," etc. The enrichment of dolomie in magnesia is
called "metasomatism." This change of "soma" is in fact a
transmutation perhaps identical to one of the bacteria-generated reactions
occurring in the genesis of raw saltpeter. The latter contains magnesium
nitrate in addition to potassium nitrate. The magnesium derives from calcium
according to the reaction Mg24 =>
Ca40 – O16.
The term "dolomite" has a precise meaning as a mineral: it is the magnesium carbonate, which is
found in varying quantities in rocks. Dolomie is the association of minerals.
Limestone almost always contains some dolomie. The rare banks of dolomie are
sought for the fabrication of cement.
In warm marine waters, plants (calcareous seaweeds) fix magnesium
calcite; Ca and Mg are not separated. These seaweeds fix the magnesium of
seawater and transmute Mg into Ca so that one always finds a mixture of these
two elements. The same applies to shells in warm waters. Dolomites are
prevalent in corals at a depth of 200 meters. Do the animalcules of the coral
have difficulty in producing a transmutation at this depth with a 20 kg cm2 pressure? Either the temperature is too
low at that level or the factors of depth and temperature both come into play.
A third possibility is that cold impedes the development of the coral – and Mg
is linked to both cold and heat Cold provokes the reaction Na + H => Mg,
Mg affording protection against cold, whereas Na ± 0 => K provides protection against heat. Cora1
beds in cold environments produce Mg; those in warmer and more oxygenated
waters are, as they approach the surface, able to easily transmute magnesium
into calcium (Mg + O => Ca) making the
coral at the surface a non-magnesian limestone.
Meanwhile, some authors* had shown aberrant analyses for
magnesium. In 1856 Lauwes and Gilbert verified that there was less magnesium in
the ashes of grass receiving magnesia salts than in plants receiving no
fertilizer. Branfield, in Continuous Creati01 (1950), writes that the
chloroplasts of plants cultured in water free of magnesium contain chlorophyll
and thus magnesium. In 1929 Gortner established that there is 4.5% MgO in the ashes of chlorophyll.
[* a) A. Ronna, Rothamsted, "Un Demi-Siecle
d'Experience en Agronomie p Lauwes and Gilbert," La Maison Rustique, Paris
1900.
b) Brochures published from 1875 to 1883 by Verlag von
Hermann, Pete Berlin.]
In the past, deeper and more precise research had shown that
magnesium is related to other elements, but no one could see the nature of the
link.
Von Herzeele noted an increase of magnesia in plants
germinated without the help of magnesium. He also thought he had found the
relation between Na and K with Mg; but he had always used Ca in a complex
solution. Because of this his deductions were contested.
Barbier and Craminade declared that "each of the Na,
Mg, an K cations present in small quantities is exchanged as if the mass of
other cations were constituted only of calcium."
The link between Na, Mg, K and Ca is thus perceived. D.
Bertrand writes that the movement of magnesium "supposes a close
correlation between the development of the micro-organisms an the development
of the plant, and this has never been studied.**
[** Le Magnesium et la Vie, P.U.F. Pub., Paris.]
In 1942 Hunter, experimenting on lucern grass, conclude(
"One often admits that K + Ca + Mg is practically constant." ] 1947
Prince, Simmerman and Beau, studying lucern in twenty different soils, say
"The most significant and simple factor influencing the amount of Mg
absorbed by lucern is the K content. If
in cases of successive culture this content decreases, the magnesium content
increases, even if the lucern grows in a soil deficient in Mg." The
authors did not even ask themselves how there could be an increase in
magnesium.
"Agronomists engage themselves in perfectly useless
expenses by needlessly compensating a presumably insufficient magnesium
content," writes D. Bertrand. He adds, "The liming can modify in
great proportions the quantities of magnesium absorbable or absorbed by the
plants."* No one could be closer
to the truth . . . and not see it!
[*Le Magnesium et la Vie, P.U.F. Pub., Paris.]
The Imperial Bureau of Soil Science in England makes the
following statement: "A significant magnesium deficiency in the soil is
undoubtedly more common than we have thought until now."
But, Bertrand writes, "It does not seem to preoccupy
anyone anymore." The agronomist does not seem to care that magnesium comes
by itself!
Endogenous
production
Magnesium is considered to be one of the most important elements
in life – not only in plants, where the chlorophyll molecule is built around an
atom of magnesium, but also in animal life.
The importance of magnesium is such that in 1960 D. Bertrand
wrote a whole book about it, called "Magnesium and Life".
Aberrant results
A) VEGETAL
The results given by D. Bertrand are completely aberrant: no
magnesium fertilizer is given to the soil, yet the vegetal matter takes away
great quantities of magnesium.
A few examples:
Mg taken
away in Kg/ha *
Wheat 12.8 (grain and straw)
Corn 54.5 (grain and straw)
Potatoes 24.5 (haulm and tubercule)
Sugar beets 37.2 (leaves and roots)
Fodder beets 35.2 (leaves and roots)
Artichoke 20.5 (head)
Cauliflower 40.9
* 1
ha = 2.471 acres
However, virgin land contains, in its arable soil, from 30
to 120 kg of Mg per hectare (ha). Regarding this fact D. Bertrand writes,
"The major part of arable lands would very quickly be exhausted, a
conclusion which this experiment invalidates." So? He does not see any
explanation.
B) ANIMAL – THE
RAT
In 1918 Osborn and Mendel made very careful experiments on
the rat. Concerning this, D. Bertrand wrote, "They could verify that the
rat requires a small amount of magnesium. But in spite of their care it was not
possible for them to prove that magnesium is necessary to a rat's life."
These results were contested, the precision of the methods of analysis called
into question. However, the most precise methods of analysis were at that time
confirming the aberrant behavior of magnesium. D. Bertrand declares, "The
experiment made on the rat proved that the latter keeps the magnesium rate of
its organism constant. Medes, Bukner and Peter fixed the Mg at 45 mg for 100
grams of fresh weight, but found that no matter how old the rat – 29, 60, or 90
days old – the amount of magnesium in its diet had a very small influence on
the amount of magnesium in its organism."*
[* Transmutation à Faible Energie, 2nd revised edition, p.
100, Maloine Pub. (Paris).]
Thus it is of little importance whether there is a
deficiency in ingestion. Here again, however, practical means for solving the
problem were seen. D. Bertrand cites Mendel, Benedict and Bogerth who say,
"A diet rich in calcium increases the elimination of magnesium and thus
increases the need for it." This last assumption is erroneous, for no one
saw that it is Ca which produces Mg and that the more Ca is added, the more
Mg will be found, as happens in plants!
C) MAN
I will state here only a few experiments made in the Sahara
with the cooperation of Prohuza, an official organization. I had been sent
there officially in 1959 by the ethnologist Jacques Soustelle. I was able to
observe the working conditions and have access to the detailed results of all
the analyses (research coordinated by D. Borrey).
The experiment took place near Ouargla with a team of petroleum
workers; it lasted six months. Here is a magnesium balance sheet (values in
milligrams, per man per day):
Fig.4 Average
variation of Mg in petroleum workers in the Sahara. (Between the two graphic curves, the negative balance sheet =
excess in excretion.)
Ingested Excreted Balance sheet
April 288 290 – 2
May 247 354 – 107
July 348 528 – 180
Sept. 5-9 198 420 – 222
Sept. 12-16 211 286 – 75
Average 258.4 375.6 – 117.2
These aberrant results were to be verified with the cooper:
of the French Marine Militaire. Prohuza, which was really interested in the problem
of living in a hot climate, made the experiment again; this time using a
physiology laboratory situated drier climate near Tindouf. The experiment
lasted eight months. Here is the
magnesium balance sheet. I will give
only general average, per man per day,
Ingested Excreted Excess
314 570 256
which equals 80% more rejected than ingested.
Thus no matter who the team of research workers or which
laboratory of analysis (first experiment by the Pharmacy Faculty of Strasburg,
second experiment by the laboratory of the Marine Nationale de Toulon) it was
confirmed that during great dry heat an organism secretes more magnesium than
it receives and in such quantities that there can be no question of an error or
a mobilization of reserves. The amount of magnesium able to be mobilized in the
human body is only 5 grams; but the August figures (from the second experiment)
reveal:
Ingested Excreted Excess
395
1047.5 652
It is quite obvious that in eight days these people would have
lost all the magnesium they could mobilize; they nevertheless
"survived" eight months.
Let me point out that the balance sheets of sodium were the
contrary, positive; i.e. in this hot and dry climate the organism was absorbing
more sodium than it excreted, and no sodium accumulation was observed. It is
interesting to know that the salt in a hot and dry country is richer in
magnesium than sea salt. That most of the waters of arid, warm regions are
salted and very: rich in magnesium.
This continuous increase of magnesium comes from sodium by
means of the reaction Na + H => Mg.
(The sodium in plasma is the source of the magnesium production in animals.) We
have the formula 1123Na + 11 H :=:
12 24Mg.
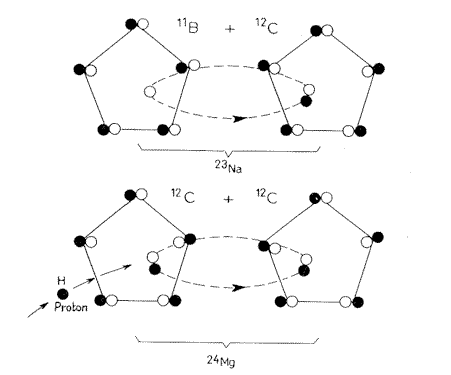
Fig.5 Passage from
sodium 23 to magnesium 24: 23Na + 1H :=: 24Mg
The occlusion of H
would take place in the boron 11B to give carbon 12C.
Note: The two parts of the nucleus are superimposed.
For diagrammatical clarity we are representing these figurations side by side,
indicating the liaison orbits by dotted lines.
Ed. Note: We advise the reader to consult all the
books about magnesium that he can find on the market. He will be able, after
reading this book, to make sense out of almost all the aberrant metabolisms of this
element. He may also obtain much information from J. I. Rodale's book
Magnesium: The Nutrient That Could Change Your Life,* from which he will learn
the multiple and important role of magnesium.
[*Prevention/Pyramid Publishers.]
VIII
MAGNESIUM – CALCIUM RELATION
There is abundant literature on calcium, but there is
relatively little systematic research available on magnesium, the central
constituent of the chlorophyll molecule (having a porphyric structure of one
magnesium atom fused with four nitrogen atoms). The harvesting of crops
involves the removal of the soil's magnesium. Nevertheless, it is rare to find
an author who advises restitution of magnesium to the soil, as Liebig's theory*
would warrant. Why is Liebig not heard? Quite simply because in most fields
magnesium is inexhaustible. There is a magnesium autogenesis, which has
remained one of the enigmas of agronomy.
[*The qualitative restitution to the soil of the quantity
taken by the harvest.]
Already in 1858 Malagut and Durocher had written,
"Although magnesium is found in almost all vegetal life, one should not
conclude that the importance of this matter in arable soil is as great as that
of lime (CaO). It has been observed that when magnesia is missing, lime can
replace it; but the opposite does not hold true."
Experiments
The following experiment was carried out: a group of animals
fed with a diet deficient in magnesium was compared to another animal group
receiving a normal diet. Since 1918 different countries have conducted intensive
research of this kind, considering the male/female factor as well, since the
latter has an influence on quantitative variations. But the direction was found
to be the same in all animals.
Some scientists varied the phosphorus, calcium, and magnesium
amounts in the diet and verified the resulting weight differences in the
animals. The result of this was an attempt to fix in the food an optimum Mg
content in any way possible; for example, with calcium (Ca/Mg), with potassium
(P/Mg), or by keeping P/Ca constant.
If the magnesium percentage is decreased to 2.5 mg for 100
grams of food (which implies the purging of the food in order to take out the
Mg normally present), the magnesium deficiency is such that rats become
rachitic, their hair dull and shaggy and their tails depilated.
Variations of Ca
in proportion to Mg
The influence of magnesium on the assimilation of carbohydrates
(vegetarian diet, large bread consumption, etc.) has been shown. The need for
magnesium in man and animals is greater whenever their nourishment is rich in
carbohydrates. That is why a generous supply is always given to animals who are
being fattened by an increased ingestion of carbohydrates.
Demolon studied the balance sheet of calcium (and phosphorus)
in dairy cows and found a negative balance sheet,* These animals secrete more
calcium than they ingest. I have made the same verification with regard to
hens.
[*Le Phosphore et ill Vie (P.U.F. 1949).]
There has been a mystery concerning the formation of the
shellfish carapace. It has been said that the animal "fixes" the
calcium of the sea, but this is another unfounded assertion.
One day my grandchildren brought me a crab that was in the
process of molting; it was a soft mass. So that it would continue to live, we
placed it in a cave containing a very small amount of seawater. The next day it
had already acquired a firmer carapace, which was completed the day after. In
approximately thirty hours a crab forms its carapace, which, for a 17 X 10 cm
size, weighs 350 grams. The calcium content of seawater is very small (on the
average, Ca = 0.042%). The molting shellfish is unprotected from marine animals
and, being very vulnerable, it hides and does not hunt.
A body analysis of the crab has shown that its
hepato-pancreas alone stocks a small amount of limestone (calcium carbonate)
before the molting, but that its carapace contains forty times more limestone
than its pancreas. Then?
We have seen that the magnesium (and potassium) found in
seawater (5% magnesium salts and 0.5% potassium salts) can give calcium and
that it is essentially magnesium, which is utilized by the shellfish to make
its carapace.
At the Maritime Laboratory of Roscoff, a crayfish was put in
a seawater basin from which limestone had been removed by precipitation; the
animal made its shell anyway.
Chemical analysis made on animals secreting their shells has
revealed that limestone is formed on the outer side of a membrane although on
the opposite side of the membrane, where matter enters, there is no limestone.
This fact has left specialists perplexed.
Of course, scientists who have been experimenting in this
field are criticized by other analysts; that is in the order of things. But the
innovator is not always wrong; nothing is perfect (the perfect being
inaccessible to man) and someone will always find a point to criticize, for
that is how progress is made. I will therefore refrain from claiming that the
methods I have used thus far are perfect. However, I judge results valuable
when they are of relative value when measured against variations obtained by
the same method.
I have accepted the research of the authors cited, insofar
as they provide strong guarantees. With this research the chemists-biologists
demonstrate, themselves, that with regard to living matter there are
inadequacies in Lavoisier's law concerning the conservation of matter. Thesis
judges concur with them, thus showing that our conclusions concerning the
failure of Lavoisier's law in the field of biology are beginning to be
officially admitted.
IX
THE LINK OF MAGNESIUM WITH CALCIUM AND PHOSPHORUS
Until now it has been impossible to refute here the classical
explanations popular among most scientists. This would have taken too much time
and would have lent a polemical character to this work, diluting a substance,
which I wanted, above all, to be objective. Furthermore, I have always supposed
that the reader knows as well as I the scholastic explanations, making it a
waste of time to discuss them.
Many of my correspondents and colleagues have told me that
it is unnecessary to discuss the arguments of "systematic opponents."
One of them wrote to me, "Expurgate your work of . . . stagnant opinions.
Make a clean sweep of ignorance." Nevertheless, I believe it will be
beneficial to give the reader, in what follows, an example of objections he may
confront from persons wishing to debate certain issues.
Various studies
concerning the magnesium-calcium link
We have seen that calcium could have had many origins, one
of which is magnesium (12Mg + 8O :=: 20Ca). Geologists believe that magnesium was twelve times
more abundant in the Pre-Cambrian era. We know that in seawater, strongly
deficient in calcium but containing magnesium, a molting crustacean can make
its shell. We also know that in the germinating seeds of certain plants,
magnesium diminishes while calcium increases. (In certain plants K decreases while
Ca increases.)
The link between magnesium and calcium has now been widely
studied by many research workers. In 1964, in one of the laboratories of the
Institut National de la Recherche Agronomique (I.N.R.A.), P. Larvor and his
colleagues* conducted an experiment with calves in order to demonstrate that
the skeleton does not develop at all when the diet is deficient in magnesium.
The calcium rate in the blood and muscles becomes too low and tetany results.
Eventually death occurs, preceded by convulsions, if the magnesium deficiency
is prolonged. Conversely, an overdose of magnesium helps develop the skeleton
and also promotes a rapid increase in weight.
[*P. Larvor et coll., Effets du magnésium sur la croissance
du veau, I.N.R.A., Paris 1964.]
A study by Dr. L. Bertrand** on spasmophilia, comprising 83
references, shows that in cases of magnesium deficiency hypocalcemia occurs,
causing tetany (spasmophilia). The administration of calcium cannot
re-establish the normal calcemia. On the other hand, a magnesium ingestion
causes a calcium increase. (Magnesium is most often administered in the form of
chloride.) It would be impossible to cite all the applications now being made
of the established fact that without magnesium the organism cannot have calcium.
I have also verified a link between calcium and phosphorus, and these
discoveries are developed in many of my books.
[**2 La Spasmophilie, edit. Les Cahiers Sandoz, Paris, June
1966.]
The link with phosphorus hasn't been investigated a great
deal by other research workers, however. Whatever was studied came to my
knowledge only incidentally, during the magnesium-calcium study. However,
despite the many experiments on the subject of the magnesium-calcium link,
some critics nonetheless declared that what had happened was either:
a) a "mobilization" of
calcium, causing it to leave the skeleton, thereby increasing the calcium rate
in the muscles and blood, or
b) a "catalytic" action
by magnesium (necessary for the "fixing" of calcium).
However, nothing of that sort occurs. Decalcification was
verified, by any means. The bone became more solid and developed in young
subjects. A catalytic action by magnesium did seem valid either, for a catalyst
remains intact at the end of reaction.
Experimental procedure
a) Animals
Forty-eight female mice weighing an average of 25 grams each
were divided into two equal lots. One lot was put aside to serve as a control.
The remaining lot was given, through an oesophagus probe, a dosage of 100 mg
per kg of magnesium chloride per day. The animals were put in different cages –
twelve to a cage. Their feces were collected in Erlenmayer type bottles. Plenty
of water was provided and food was given by cramming. Three times a day each
animal received 1.5 ml of mash made of crackers reduced to a powder with water
added (1 gram of powder for 2.5 ml of water).
The experiment lasted five days. On the sixth day the
animals were sacrificed with ether after a twenty-four hour fast.
b) Mineralization analysis
Both batches of mice were dissolved in nitro-sulfuric acid.
The collected excreta were added to each corresponding lot of mice. After mineralization with perchloric acid,
the phosphorus content was determined by a colored reaction employing sulfuric
aminonaphtol sulfonic-molybdate. The calcium was first measured by direct
complexometry in raw solution.
c) Results
Control Treated
Total weight before experiment 614g 604g
Total weight after experiment 628g 620g
Calcium weight 1.87g 2.48g
Phosphorus weight 1.83g 2.40g
Fig. 6 Ca and P
contents in 24 mice, with and without magnesium supplement.
d) Commentary
In order to facilitate the comparison, I have arranged that
the total animal weight be equal for the total of each of the two lots,
assuming that P and Ca are proportional to weight. The treated lot was 10 g
lighter at the outset. If the difference is rounded off to the 1/6Oth and if
the control mice weighed as much as the treated ones, we would have for the
control lot:
Ca = 1.84
P = 1.80
Assuming an equal weight for the two lots, the receivers of
magnesium saw an increase of
2.48 – 1.84 =
0.64 g (or 34.78%) for Ca
2.40 – 1.80 =
0.60 g (or 33.3 %) for P
e) Conclusion
Hence we see clearly and beyond any mathematical or
statistical contestation that calcium and phosphorus increase when magnesium is
given in overdose, and that this occurs within a few days. If an error had
resulted from the analytic method, it would have taken the same direction for
the two lots and the difference would have remained the same. We can therefore
assert beyond the shadow of a doubt that magnesium was the source of the rapid
increase of calcium and phosphorus.
Let us recall that in the organism certain mechanisms often
enter into play: calcium and phosphorus may have other origins. (Calcium may
come from silicon and potassium, phosphorus from sulfur and nitrogen.) Due to
the metabolic complexities of the animal, complementary experiments were made
on grains and microorganisms, the sole purpose being to clarify Nature's
mechanisms.
The following objection, among others, was given to me: the
weight increase in the animals receiving magnesium resulted from their being
bloated with water. If that were the case it would follow that those receiving
Mg would have been thirstier. Observation shows, however, that magnesium
chloride does not cause thirst; one may take it regularly and still remain
"as thin as string." The Mg++ ion does not cause retention of
water in the tissues; it is Na+ that provokes water retention at the level of the renal
tube, hence the impression of thirst. That is why taking sodium chloride is not
advisable in a case of oedema.
To the objection that the experiment was rather short, it is
easy to answer that an experiment's duration is proportional to the subject's
speed of metabolism, thus to the subject's growth rate and weight increase. In
the case of microorganisms, we have seen that an experiment may last 2 days;
for mice, weighing an average of 25 grams, we adapted our research to 6 days. Larvor
experimented on calves weighing 50 kg before the experiment. With an overdose
of Mg, they weighed 75 kg after 4 weeks. But with magnesium deficiency they
weighed only 65 kg.
Ed. Note: A few other experiments concerning the
magnesium-calcium link will be given, with commentary, in the chapter on
nutrition and medicine. I placed them thus because I felt that the latter
chapter would attract the attention of the layman who, at this time, is drawn
to subjects related to the improvement of his health.
X
PHOSPHORUS
Phosphorus is very often linked to sulphur; it is found in
amino acids. A vital element of the highest importance, it not only has an
important role in the bones, in gray matter, and in the envelopes of neurons, but
it is in fact a constituent element of the nucleic acids.
In the chain of DNA molecules a phosphoric group alternates
with a deoxyribose. Phosphorus is thus one of the constituents of
deoxyribo-nucleic acid, which carries the hereditary genetic code.
It is also found in ribonucleic acid (RNA). Phosphorus and
sulfur, which are major elements of life, are linked in the relation P + H <=> S, which allows nature to go from
one to the other in case of a deficiency.
The reader will recall these previously shown relations:
Na + H => Mg
Na => Li + O
Mg + Li => P
Mg + O => Ca
These reactions explain the
P/Ca equilibrium and the
chemical combinations in the form of calcium phosphate occurring in the bones.
However, the organic phosphorus of nucleic acids seems also
to derive from sulfur, which is a "condensate" or "doublet"
of oxygen.
The phosphorus and sulfur link is brought out in the
following remark by A. Voisin: "Giving mineral phosphate to the soil does
not modify the phosphate content of a cereal, but increases its thiamin
content" (Vitamin B1).*
[*"Sol, Herbe, Cancer," La Maison Rustique, Paris
1959.]
The P /S balance shows why the organism cannot accept a
local excess of P and explains the brutal effects of some phosphoric esters
used as insecticides.
It is certain that nature has other methods of producing
phosphorus.
The layers of phosphates in ores are calcium phosphates.
This is not astonishing since P and Ca have a common origin, but the fluorine
content of the ores is also considerable (F/P2O5 = 9%, on the average). It seems that
the following reaction occurred:
P31 <=> C12 + F19
The phosphorus "birth" was never explained; plants
make it, and A. Demolon and A. Marquet say, "The essential characteristic of
phosphorus is its fixation and its concentration in the superficial zone of
cultivated soils, always noticeably richer underground."**
[**Le Phosphore et la Vie, P.U.F. edit, Paris 1949.]
Mr. P. Dahiez from his laboratory of soil study points out a
reaction opposite in direction from the one cited by A. Voisin. He writes,
"Some soils treated with derivatives of sulfur, when analyzed, gave high
percentages of phosphorus, whereas other soils evidenced a phosphorus
deficiency." He also cites for us other "quite curious facts whose
explanations cannot he given by the data ordinarily yielded in agronomy."
In the animal organism the fabrication of phosphorus may follow
another process, deriving from the chlorine of the blood's sodium chloride:
Cl – O => F
F + C => P
This is a possibility. However at this time we do not have,
even from exceptional cases, a cross-reference confirming that this reaction
is possible except in the presence of fluorine in phosphate lies. This fluorine
possibly comes from seawater.
Fig. 7 Diagrams of
the nuclei of phosphorus and sulfur. One recognizes, on the left (solid lines),
the two nuclei C (2.C =>
Mg). The isolated vertical plan on the
right is Li, and Mg + Li =>
P. Li, linked to the set, can receive
H. When H is added we have P + H => S. It is clear (dotted lines) that the set is also
equivalent to 2.0 – H =>
P. If H is added the result is 2.0 => S.
Phosphorus deficiencies impede plant development, preventing
protein molecules from being formed. So, because the amino acids cannot be
formed, phosphorus was believed to be linked to nitrogen. The establishing of
this link rests upon the fact the ratio of the total nitrogen in phosphorated
fats (phosphatides) is constant in the different plants. In fact, there is no
direct link. It is a phosphorus deficiency, which impedes the formation of the
amino molecules, also causing a reduction of the other elements. But it is also
the sign of a deficiency in elements, causing the plant to make up for the
deficient phosphorus.
The phosphorus-calcium link is commonly known. When much
phosphorus is produced in an animal, much calcium is also produced.
Negative balance sheets of P and Ca are also cited by
Demolon, who indicates that the weights of these elements given for dairy cows
"are noticeably inferior to the quantities of these elements which leave
the animal's body with the milk"* (approximately 3 grams of P and 3 grams
of Ca for every liter of milk). But the cow has other needs for these elements
in the maintenance of its body, and it excretes through the urine and fecal
matter quantities, which, together with what the milk takes away, exceed
significantly that which food brings to it. A declaration such as "The cow
borrows calcium and phosphorus from its own reserves" is unacceptable. One
good dairy cow, weighing 700 kilos and giving 30 liters of milk a day, needs
111 grams of P per day (3 grams per liter of milk + 3 grams per 100 kilos of
weight). Another cow was given 98 grams of P per day, which is a daily deficit of
13 grams compared to that of the first cow. In 100 days it would have taken
1,300 grams of P, although its whole body (skeleton, flesh, blood) contained
only 4500 g. It is obviously impossible to reduce the amount of phosphorus to
such proportions. There is, then, an endogenous production of phosphorus
(which is also true for calcium, and in the same relative proportions).
[*Le Phosphore et la Vie, P.U.F. Pub., Paris 1949.]
Application for
Geology
In some parts of my books references are cited pertaining to
the simultaneous presence of Li and P (in plants, bones, ores). This is surely
a case of fortuitous association.
If P – Li => Mg, we have seen that Mg and P are precisely
linked to such an extent that if the organism excretes more P than it receives,
it also excretes more Mg than it ingests. The negative balance sheets of P
always corresponded – with the same subjects, within the same time – to the
negative balance sheets of Mg. The average on the observed negative balance
sheets was 134 mg of phosphorus excreted per day per man. These balance sheets
were calculated simultaneously with the negative balance sheets on calcium. The
calcium represented 321 mg/day per man, whereas the negative balance sheets of
magnesium averaged 163 mg for the same days.
The opposite is equally true: whenever there is a positive
balance sheet for Mg, there is a positive balance sheet for P and Ca, showing
not an association but a filiation. P
is born from Mg, but only in the presence of Li, which, in organisms of higher
animals, comes from the sodium of the blood plasma (Na – O => Li).
P can also be formed in magnesium rocks by
"frittage" of Mg, with Li coming from potassium (K – 2.0 => Li), thus indicating
that phosphorus can be found in the soil without sea water being present.
But we also saw in the formation of the dolomies that Mg can
derive from calcium (Ca – O => Mg). A lie of limestone influenced, I believe, by
bacteria, can give Mg. If there are alkali (Na or K) present, there is a
possibility of producing phosphorus which, along with calcium, can give calcium
phosphate.
This is another method of forming calcium phosphates, and it
is probable that the two types of lies (marine and continental) exist.
The phosphorus in lies is linked to the presence of
bacteria. This seems to show that P has an endogenous formation due to a
specific enzyme yet to be investigated.
This enzyme needs Mg, or at least it seems that Mg is the
most abundant origin of P. Furthermore, the phytin in plants is a magnesium
salt of a phosphoric ester.
Research is being done in many laboratories to clarify this
formation of P. Here are those
discoveries, which were brought to my attention after I had published my discoveries.
a) In the germination of seeds there is a P variation
between the seed and the young plant having germinated without a P supply.
b) During the activity of yeasts the P content also changes.
c) A seaweed rich in calcium (where, for example, Ca/Mg = 6)
has been sown with bacteria in order to study the P genesis.
Phosphorus
variation in the germination of seeds
The variations of organic forms during plant maturation is a
solely chemical operation, a rearrangement of atoms and molecules. Y. Colin made a study on sunflower seeds
during maturation.* He saw some aspects of the phospho-organic compounds'
variations in the seeds and in wheat germ. We will only expose the results of
the total phosphorus variation during germination, which is a case of the
transmutation of one element.
[*Y.
Colin, "Evolution des Composés Phosphoriques au Cours de la Germination,"
Bulletin Soc. Chim. Biology, 1934.]
Methods of dosage
There is no room here to condense the 84 pages of the Colin
thesis, but it appears useful to make a rapid survey of the difficulties of
such a problem. With a reaction which appears as the separation of a compound
one runs the risk of blocking, in a totally insoluble form, another compound no
longer able to be dosed. This is why research on the separation of various compounds
has given birth to a different problem.
Phosphorus is found in the form of phosphoric esters, such
as the phospho-lipids and the phospho-amino-lipids. In the phospho-lipids one
finds lecithins and, most of all, the derivative of glycerophosphoric acid.
Also present are the phosphoric esters of sugars such as monophosphoric ester,
disphosphoric-hexose, monophosphoric-hexose, etc.
The phospho-lipids vary during germination. W. Maxwell, in
1891, may have been the first to put this into prospective. Others followed,
using different seeds. In 1910 Miller verified that in sunflower seeds the
lipidic reserves decrease from 55.6 to 13.5% after 13 days. In 1902 Iwanow found that in vetch the
lipidic phosphorus, which is 11.6% of the total phosphorus present at the
beginning of the germination, is only 6.6% after 20 days. The protein content
falls from 52.5 to 13.7%.
In 1912 Bernadini and Morelli showed that during the germination
of 500 seeds of wheat the phosphotidic phosphorus increased from 19 mg to 55 mg
in the light, whereas it disappeared completely after a few days in the dark.*
[*Colin, "Evolution des Composés Phosphoriques au Cours
de la Germination."]
Experiments in the light
In an experiment done in the Museum of Natural History
greenhouses, half of the basins received light and half were covered with
black paper. The greenhouse temperature at the moment of maximal insolation
was 30° C (in May). The rapidity of germination made possible the first harvest
after two days, the second after three days, the third after six days, and the
fourth after nine days.
Here are some figures, in mg, on sample gatherings
consisting each time of 400 seeds of lentils, which were germinated, in the
light:
Lipidic Nucleic Phytinic Mineral Total
Before germination 12.67
mg 10.4 mg 50.63 mg 18.30
mg 92.00 mg
After two days 11.85
9.76 48.38 21.81 91.80
After three days 11.35 11.15 38.31 30.84 91.65
After six days 10.25 12.50 12.64 54.61 90.00
After nine days 9.45 15.25 0 62.30 87.00
'Thus along with the variations in the different compounds
of phosphorus, an important fact is established: the total phosphorus
diminishes up to 5.43 %.
Fig.8 Variations of
total P in the germination of lentils (May).
Another experiment was conducted from the 23rd of June the
4th of July (12 days). This time it was conducted on portions of 325 seeds of
lentils, but on porous paper imbibed with double distilled water. The average
weight of every portion of lentils was 26.63 grams.
P
Total
June 23 (before
germination) 112 mg
June 27 (after 5
days) 111
June 30 (after 8
days) 110
July 4 (after 12
days) 105
There was a 6.25% decrease in phosphorus (experiment made in
the light).
All experiments concerning the total phosphorus variation
during germination converge and one must conclude, beyond any dispute, that
there is a certain disappearance of phosphorus, varying with the particular
species of seed, the germinating conditions, and the time of year – but always
in a highly significant degree. No error can be imputed to these results since,
whatever the methods, the laboratories that were employed had the most modern
equipment, the operators were different, and the result always pointed in the
same direction.
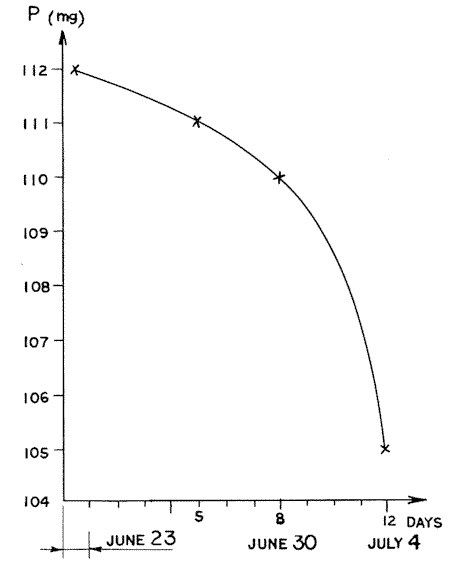
Fig. 9 Another
variation of total P in lentils germinated in the light (summer).
XI ABERRANT METABOLISM OF SOME LIVING
ORGANISMS
a) Life sustained with clay: a shrimp living in the dark
In a medium entirely composed of humid clay, life is
possible. The humid clay is compact, thus air cannot enter. This clay is
impermeable to carbon and oxygen. Oxygen can, nonetheless, be produced by
dissociation of the water molecule – and we understand how plants use the
oxygen from water. The oxygen that plants reject into the air does not come
from the absorbed air, of which the plants would have retained the carbon of
the available carbon dioxide. Would the living organisms in the clay then be
able to extract their oxygen? This question remains unanswered, but let us
admit such a possibility.
Another problem remains to be solved: carbon is necessary to
any organic life. Animal and vegetal tissues are composites of carbon. They
develop by making new tissues, thus by taking carbon. Furthermore they breathe,
ultimately rejecting carbon dioxide (CO2).
This means that the carbon supply must be renewed because animals do not take
back the carbon dioxide, which they excrete – it is a poison for them. In clay,
animals avoid these rejected gases by moving continually from one place to
another. The rejected carbon dioxide, being at a pressure superior to that of
the ambient gas, is little by little diffused. The carbon dioxide on the
outside cannot enter because of the great pressure inside the clay.
It has been known for a long time that living organisms
inhabit clay while having no organic supply from the outside. This fact has
intrigued research workers and an important study was made in a laboratory
installed in the cave of Moulis, France; the results were published in several
French scientific magazines.
Let us note the case of the Niphargus shrimp, a small animal
half-an-inch in length that lives in the clay of caves. If a shrimp is given
organic matter (meat, etc.), it vegetates and dies. It also dies if it is not
kept in humid clay. Experiments have shown that it grows normally in pure clay
to which nothing has been added. Research workers therefore thought that the
shrimp lived on clay and nothing but clay, an impossibility according to the
laws of biochemistry. Actually, it cannot live thus in clay alone, but this
clay contains microorganisms which work for the shrimp, making vitamins,
various mineral products, nitrogen, phosphorus, and calcium, etc.
b) Earthworms
The earthworms' role has long been ignored. They were
thought to be good only for the mechanical function of making the soil lighter.
Notwithstanding, some research workers have demonstrated that annelids modify
the chemical composition of the earth.
In his "Treatise on Microbiology of the Soil"*
Pochon, of the Institut Pasteur, gives various experimental results. The earthworms
increase the quantity of limestone in the soil. Their glands excrete CO3Ca so that the pH of soil containing
earthworms increases. Earthworms are most abundant in neutral or slightly
acidic soil and can be found in good soil by the hundreds of thousands per
acre. Some authors declare that each worm ingests l/l0th of a gram of earth per
second, which is three tons per year. Darwin gives a higher figure, but one
should be careful with such calculations since earthworms have resting periods
in winter and in dry seasons. Other believable figures, which resulted from
observations made in England, indicate that a field of earthworms rejects an
average of 57 tons/ha/year (23 tons per acre per year): the equivalent of four
spreadings of farm manure per year. But this constitutes only the amount of
earth rejected on the soil's surface. One cannot deduce from this the exact weight
of earth having passed through the digestive tube of each worm. Compared to the
surrounding soil, these rejected excrements were five times richer in nitrogen,
two times richer in calcium, two-and-a-half times richer in magnesium, seven
times richer in phosphorus, and eleven times richer in potassium.
[*Dunod Publ. (Paris 1954).]
c) To live on an iron wire
In one of my books I noted the curious case of plants
commonly called "Spanish Moss" which grow in green masses, most often
on copper wires. Their botanical name is Tillandsia. They are usually found in
humid, warm regions on telephone wires. They adhere to the wire by means of
viscous disks and can also cling to branches, dead trees, and rocks.
According to Pfeiffer, the analysis* of these plants shows
that they contain approximately 17% Fe2O3 in their ashes, but little noticeable
amounts of copper, although the analysis was made on plants growing on copper
wires.
[*Fécondité de la Terre, p. 152-156.]
These curious plants grow without roots; they have no
contact with the soil. That is what intrigued everyone: where did they obtain
the minerals revealed in the analysis? One may suppose that water, carbon, and
nitrogen were obtained from the air, but what of the other minerals?
Everything that has been written about the origin of these
minerals is but groundless assertion. It has been proposed that the rain brings
them – in minute traces, of course – and that they accumulate with time. Others
say that they come from dust. All this to respect the dogma of the non-creation
of matter. But these hypotheses are unsatisfactory.
H. Friedel, in his work "Les Conquêtes de la Vie",
also writes about these plants: "I must confess that all my notions about
the vegetal have been overthrown in Antibes in the Hennessy garden, where I saw
Bromeliaceae develop in the air on an iron wire. ... In order to adopt oneself
to such a 'territory' one must really be a vegetal."**
[**Larousse, 1967, p. 128.]
What allows us to reject the above hypothesis is that these plants
can acclimatize themselves in a greenhouse. In Alsace, France, successful
experiments have been made on copper wires in greenhouses. The hypothesis given
about atmospheric dust and rain is then of no value. In the greenhouses there
was only air, sun, water vapor from the humidity of the air, and symbiosis with
copper. No copper was found in the plant, but iron was found. No chlorine was
in the air either, although there was some in the plant.
There are other examples, too numerous to list. Here is one
that Pfeiffer cites: "The Sarothamnus vulgaris is quite an amazing plant.
It is particularly rich in lime ... moreover, its roots secrete lime which is
deposited in circles on the bark, so much so that it is the plant which
provides limestone for the soil. But the Sarothamnus vulgaris grows almost
exclusively in siliceous fields. That is why it is particularly appropriate for
the preparation of fallow fields!"*
[*Fecondite de la Terre p. 157.]
XII NITROGEN
When I proposed that nitrogen could become carbon monoxide
and commented on experiments demonstrating this, I was sometimes told that I
had given a rather "audacious explanation."
It would take too long to set forth all the reasons that led
me to the identity 2N14 =>
Cl2 + O16. Such an undertaking would require a
lengthy discourse citing all the inconsistencies in previous attempts at
explaining the aberrant behavior of nitrogen in biology.
I shall therefore limit myself to a few brief examples of
experiments showing the relation of nitrogen to other elements.
Aberrant metabolism of nitrogen
I shall cite a few examples, some of which have been
borrowed from Terroine,* the author of three important volumes dealing with the
metabolism of nitrogen.
[*Métabolisme de l' Azote, edit. P.D.F., Paris 1933.]
The first volume contains a chapter entited "Do
Nitrogen Leakages Occur?" In addition to his own experiments, Terroine
cites 51 articles by different authors.
1) Animals
If a dog is given 15, then 30, and finally 45 grams of
nitrogen per day (measured according to the weight of ingested meat), its
intestines reject 0.30, 0.55, and 0.67 g per day, respectively.
Hence, with increased ingestion there is a proportionally smaller
excretion of N. Thus the amount of nitrogen present does not depend solely on
the food regimen.
VERIFICATION
To confirm this, numerous experiments have been made. They
are related by Terroine:
A small portion of an animal's intestine was emptied and
then ligatured. After a few days this part, which could not have received
anything from the intestines, contained an amalgam of nitrogenous substances.
There had thus been a nitrogen production on the inside wall of the intestine.
Persher gave a dog 2.33 grams of nitrogen per day, but the
dog excreted 3.70 grams every day.
In the case of man, Seller decreased the ration of ingested
nitrogen on each successive day. He began with 4.30 grams, arriving after eight
days at 3.67 grams. But the quantity rejected in the stools changed only from
5.75 on the first day to 5.04 on the eighth day. "The rejection is always
superior to the ingestion," says Terroine, adding, "but there is
more. The examination of the above data not only proves that there is a persistent
nitrogen loss but also that there is a quantitative constant of this
loss." He concludes that there is certainly an "endogenous
metabolism of nitrogen."*
[*Terroine, Métabolisme de l' Azote, edit. P.D.F., Paris,
t.I., 1933.]
OTHER EXAMPLES
A man given 0.3 grams of nitrogen per day rejects, through
his intestines, an average of 0.5 grams. A pig ingests 0.2 g/day and rejects
0.3 g through the intestines. A man who has been fasting for ten days still
rejects 0.3 grams of nitrogen through his intestines.
DISAPPEARANCE OF INGESTED
NITROGEN
With a normal regimen the endogenous production of nitrogen
does not show up in the balance sheets since the latter are always positive
(i.e. one ingests more nitrogen than one excretes). This factor has made the
balance sheet figures suspect, for classical science has found it impossible to
discover where the excess goes; obviously there is not a lifelong accumulation!
There are unrevealed "leakages" of nitrogen and, despite thousands of
experiments made by many scientists, this has remained unexplained.
A FEW EXAMPLES
The following experiment was made with a group of rats. It
was established that each rat contained 5.96 g of nitrogen. The group of rats
was put on a fast of quite long duration. Every day their fecal matter and
urine were collected. (A rat does not perspire, thus there is no elimination
from the skin pores.)
The animals were sacrificed in order to measure the total
nitrogen of their bodies; the excretions were then added. The total N weight remained
0.55 grams less per animal than the nitrogen weight present at the beginning of
the experiment. The rats had lost an average of 10% each; (one of them even
lost 55%).
A rat was enclosed for two months in a sealed tube
containing air and a chorella culture; nonetheless the atmosphere's nitrogen
content diminished a great deal, a phenomenon, which is incomprehensible
according to classical chemistry. The oxygen increased. In this case, the
nitrogen had been used in the form of C + O.
ENDOGENOUS PRODUCTION OF
NITROGEN
We have seen that the greater the amount of ingested
nitrogen, the less the proportion rejected by the fecal channel. This shows
that nitrogen disappears through the digestive channels.
Conversely, if food becomes deficient in nitrogen, the
intestines' endogenous production of nitrogen increases as if by a defense
reaction on the part of the organism. Hence intestinal action vitally needs
carbohydrates. The body grows thin due to the disappearance of carbohydrates,
which have been transformed into excreted nitrogen.
Let us totally suppress nitrogen ingestion by administering
carbohydrates absolutely devoid of nitrogen-sugar, for example:
a) A dog put on a fast excretes 2 grams of nitrogen per day.
When given 85 grams of sugar, he excretes only 1 gram of nitrogen per day.
After a few days he is given 120 grams of sugar per day; he then excretes only
0.5 grams of nitrogen.
b) A man put on a complete fast rejects a total of 11.9
grams of nitrogen per day (of which 1/5 to 1/10 is eliminated through fecal
channels). When he is given sugar he excretes only 6.3 grams of nitrogen per
day. From this we learn that the organism does not have to take carbohydrates
from the reserves of its tissues if its intestines supply what it needs in
order to make nitrogen. No longer ''hungry for nitrogen," it produces it
in smaller quantities (providing only what it needs for local functions). These
smaller quantities do not create a strong need for carbohydrates.
It is thus clear that the only valid explanation is a
transmutation from carbohydrates to nitrogen, and vice versa.
c) If this reasoning is correct, an animal receiving a small
amount of nitrogen would then produce a lot of it! In fact carnivorous
animals, which receive much nitrogen, do not produce it and excrete very little
of it. What they ingest disappears for the most part in their organisms, being
utilized for their nourishment, contributing to weight gain, i.e. enabling them
to produce carbohydrates. The balance sheets here are positive: one no longer
finds nitrogen in its own form.
Conversely, herbivorous animals, whose nourishment contains
a small amount of nitrogenous substances, are great producers of nitrogen: a
two-year-old ox (herbivorous) excretes 13 times as much nitrogen as a man
(omnivorous) of equal weight.
AGENTS OF TRANSMUTATION
By which means do the intestines create what is called
"nitrification" and "denitrification" (words which in
reality ill define transmutation)? We
know that the bacteria of the intestinal flora are the agents. It is false,
however, to say that all bacteria remove nitrogen and, when food-containing
nitrogen is lacking, use the nitrogen in the air. (This hypothesis has been
given by a few authors who neglected to measure the quantity of air in the
intestine and its speed of renewal.).
If this endogenous production of nitrogen were derived from
the air and were fixed organically in the molecules of the fecal matter, the
process would occur regardless of the nitrogen content in the food. However,
we have seen how the endogenous production of nitrogen is linked to
carbohydrates and is not directly dependent on nitrogen ingestion.
Bacteria are the agents of the transmutations of nitrogen into
carbon and oxygen, at the level of the intestinal wall. Other bacteria are
responsible for the opposite reactions.
2) Plants
Many books have been written about nitrogen. One of them,
"Respiration and Nitrogenous Metabolism" by A. Moyse, is devoted to
the leaf and cites a great number of references. Here, too, remarks are cited
showing that the relation between carbohydrates and nitrogen has long been
perceived:
Borodin (1876-1878) estimates that "there is a
decomposition and a continual regeneration of proteins," a statement which
was to be confirmed by Gregory and Sen in 1937.
Among the most interesting and memorable experiments Moyse
cites are those related to the stems of detached leaves, which have been placed
in the dark in a controlled medium. For some time, these leaves continue to
live. However, after four days a decline is noticeable. The decline accelerates
after the sixth day; in such cases death usually occurs after ten days.
In summarizing the results of a few experiments made by
Moyse on the stems of wheat, buckwheat and sorrel leaves, we have the following
data:*
– total nitrogen (solely organic) before the operation:
13.64 mg
– total nitrogen at death: 22.8 mg, in the form of mineral
nitrogen (NH3).
Thus when death occurred there had been a 70% increase in
nitrogen. This increase could not have resulted from a passage of organic
nitrogen to mineral nitrogen. Furthermore, 82 mg of carbon disappeared. (The
conditions of the experiment do not allow us to determine where the carbon
went, but it is clear that nitrogen was produced.)
[*Moyse, Respiration et Metabolisme Azoté (de la Feuille),
edit. Hermann, Paris 1950.]
Moyse points out another phenomenon concerning the nitrogen
increase: "The direct origin by liberation of the pre-existing amides in
the proteins and the indirect origin by conversion of the aspartic acid are not
sufficient to explain this increase."
It can be understood, according to the preceding
experiments, how manure may be a great source of ammoniac. There is not only a
loss of proteinic nitrogen but also an endogenous formation of NH3 from the carbohydrates of straw and
cellulose.
Zaleski (1897) remarked that "leaves can form proteins
even in darkness, and parthenogenesis requires only the presence of high
quantities of soluable carbohydrates." Light facilitates proteogenesis,
not only because of carbohydrate enrichment by photosynthesis, but also
because photosynthesis is accompanied by O2
at the level of the protoplasm. Darkness favors loss of protein because it
causes the O2 pressure to
diminish.
When exposed to light the leaf rejects oxygen by
chlorophylian action. The oxygen pressure is thus stronger in the leaf than in
the air, and that is why the oxygen leaves. In the dark it is the opposite: the
leaf absorbs oxygen; there is only respiration.
This interaction with oxygen is also observed in the fact
that proteins are formed in young and growing tissues, which are rich in
oxygen. Hence the N content varies from the roots to the leaves, according to
season and amount of light.
It seems useful to insert at the end of this chapter a few
more clarifications concerning the mysterious accidents by oxicarbonaemia.
This subject was treated previously in the chapter "Aberrant
Observations," but was not dealt with completely.
Transmutations of
N2 and oxicarbonaemia
without respiration of carbon monoxide
3) Recapitulation of experiment
In 1955 mortal accidents occurred in Paris due to carbon monoxide
poisoning. Official laboratories made a systematic study on 42 welders from
different factories. The investigation, which lasted four years, confirmed that
oxicarbonaemia always struck this type of worker.
The CO analysis was made by different methods. Here are some
results in cm3 of CO per liter
of blood. Please recall that the safety limit is 4 cm3/l, that oxicarbonaemia is obvious at 10
cm3/1, and that the danger
point is 15 cm3/l.
Factory I
A worker with 19
cm3/l.
Factory II
Worker A = 15 cm3/l
Worker B = 9 cm3/l
Worker C = 6 cm3/l
Factory III
Worker K = 14 cm3/l
Worker N = 12 cm3/l
Worker P = 14 cm3/l
Worker R = 15 cm3/l
Factory IV
Worker D = 14 cm3/l
Worker E = 11 cm3/l
Worker F = 14 cm3/l
Factory V
Worker G = 13 cm3/l
Worker H = 14 cm3/l
Worker L = 12 cm3/l
Worker M = 9 cm3/l
Worker S = 10 cm3/l
Worker T = 11 cm3/l
Worker U = 7 cm3/l
Worker Y = 4 cm3/l
Worker Y of Factory V was a manufacturer of sheet-iron working
a few yards away from the solderers and oxycutters. Therefore he did not
inhale the air, which came in contact with the incandescent sheets.
This factor indirectly confirmed that only the air, which
had passed over the incandescent iron, was causing the endogenous production of
carbon monoxide.
The possible explanations of this phenomenon are numerous, I
know, but none of the classical explanations proposed has been verified (e.g.
by air pressure, oxygen pressure, etc.).
I immediately suggested that here was a new aspect of nitrogen
metabolism. There is oxygen in CO and also in air. However, in the former there
is C and in the latter there is N2. My supposition was that an unknown
biological mechanism at the level of the red blood cell modifies the nitrogen
at the level of the atom's nucleus, so that a group of two nuclei of nitrogen
molecules modifies the internal condition of the nucleons. (The nitrogen of the
air becomes metastable by licking the incandescent iron, but it remains
nitrogen; that is why it was impossible for the chemist to find carbon
monoxide in the inhaled air.) I wrote:
2N14 =>
C12 + O16
Other observations regarding the link between nitrogen
and carbon-plus-oxygen
The N2 and
CO link was verified long ago. The reader will recall that these two have
noticeably the same molecular mass and a few common physico-chemical
characteristics.
In the reactions I have observed, the nitrogen has been in
the molecular form N2 and the
oxygen in atomic form, although there is always O2 in vitro.
It is very interesting to compare these results with the
observations made by artificial satellites. Oxygen (O2) dissociates rapidly under low energy
(ultra-violet) radiations. In the thermosphere at an altitude of 120 km, N2 is four times more abundant than O2.
At 300 km 99.5% of the O2
is dissociated into atomic oxygen, whereas the nitrogen (N) at this altitude is
only dissociated at 1 to 2% as compared to N2.
The dissociation is total only at an altitude of 1000 km, where the ionizing
actions are considerable. (Above 1,000 km there is helium and above 2500 km
there is hydrogen.)
In other works I have shown that the energies situated in
the ultra-violet (which is of medium energy) cause the nuclido-biological
displacement of oxygen. Plant and animal enzymes thus emit an energy equivalent
to that of a photon associated with ultra-violet. This would explain why in
nuclido-biological reactions oxygen is never in the form of O2 but is always in the form of O, whereas
there are no nuclido-biological reactions with N, but only with N2. (This is at least true in everything I
have observed thus far.)
Of course, not everyone immediately agreed with my conception
of the phenomenon of transmutation. M. Loeper, a French specialist very well
known for his studies on pathological cases concerning the endogenous
production of carbon monoxide, thought that a pressure reduction at the level
of the air cell (pulmonary alveolus) was the cause of bad oxidation in the
blood, thus causing the production of CO instead of CO2.
Loeper's position of authority did not prevent Professor
Desoille from concluding in Archives of Professional Diseases (July-August
1963) that there is "no correlation whatsoever between the partial
pressure of oxygen and the carbon monoxide in the blood."
Thus it was once again confirmed that the pressure of carbon
monoxide in the blood is not due to the presence of oxygen.
XIII SULFUR
Sulfur appears as a "fritting" of two nuclei of
oxygen. The most abundant form of sulfur is S32
(2.O16 =>
S32).
Let us remember that the O2
=> S relation explains the very
corrosive role of sulfur at high temperatures. It is the equivalent of a strong
oxidation. Sulfur and oxygen are two chemical aspects of the same nuclear
origin, so that when there is – at least in biology – strong oxidation in the
young and active cell, there is a concentration of oxygen. This explains the
presence of sulfur in the fast biological reactions of oxidative-reduction
(appearance and disappearance of O). It will be necessary to ascertain whether
this fact can be applied for the detection of cancer.*
[*It is the same for the increase of K, which results from
O: Na + O ==>
K, and has been detected in cancerous tissue.]
The biological sulfur (O2)
enters into the constitution of the essential amino acids. There is from 0.3 to
2.4% S in proteins. This is also the case in the egg's development process.
This biological sulfur becomes mineral sulfur when it combines with H, as when
an egg dies or rots.
In cellular metabolism five sulfured coenzymes have been
identified. Sulfur is the element of transition. Four of these coenzymes come
into play in the metabolism' of carbohydrates, lipids, and proteins.
Sulfur (O2)
is an element as vital as nitrogen (C + O), fabricated by plants as well as by
some living organisms. The latter has been experimentally proven in the case of
plants and can be easily confirmed for animal cells, for example in the hen's
egg. The identity O2 =>
S can also be verified in mineral chemistry: everyone knows that metals burn
not only in sulfur vapors but in oxygen as well. The same is true of carbon.
(There is thus CS2 with S and
CO2 with O.)
Besides discussing the "production" of sulfur by
certain plants (watercress, cabbage, etc.) and some mushrooms, we should note
some facts concerning the production of mineral sulfur.
When water is spilled on incandescent charcoal, one
"smells sulfur." Although it
is said to be a chemical reaction that provokes the release of the sulfur contained
in the charcoal, it can be a condensation of oxygen.
When lightning strikes close by, one also smells sulfur.
Scientists know that electric discharge can give ozone (O3), but doesn't it also produce SO2?
The gas of Lacq, France, contains 18% sulfured hydrogen,
which must be purified, thereby losing all its H2S. It then goes through a dryer, after which it is again
found to contain some sulfured hydrogen – as if sulfur had been produced in the
dryer. This fact has remained unexplained.
According to my thinking, here is what happens (hypothesis
to be verified): the gas is heated in order to dry it; the water dissociates in
part into H2 and O. There is
thus a possibility of "frittage" (O2
=> S, which is found in the presence
of 4H), hence the possibility of a chemical combination H2S which liberates 2H. However, S is produced, just as when water
is spilled on incandescent charcoal.
Sulfur is found in glutathione, in the Bl vitamins, etc. It is often accompanied
by phosphorus, for the organism transforms one to the other. This phenomenon
will lead to the revision of many pharmaceutical products, since sulfur enters
into the composition of many organic products such as sulfamides, penicillin,
etc.
In mineralogy, the composition of metals includes sulfur or
oxygen (O2 => S). Lead, zinc, copper, and mercury are
most often formed by sulfurs in the earth's depths (by pressure?) and oxides on
the earth's surface. Likewise iron, calcium, etc., often have S or O
compositions.
There are many cross-references which show that the probable
origin of sulfur is oxygen, that it is a "condensate" of two atoms of
oxygen, and that in nuclei acids the biological reaction is O + O => S.
In previous books I have cited an experiment consisting of
the production of sulfur with thiobacilli. For this effect the inside of a test
tube is rubbed with a wadding soaked in sodium-thiosulfate. The quantity of
sulfur thus deposited is insignificant. The thiobacilli and an appropriate
nutritive solution containing no sulfur are then put into the tube. The
thiobacilli proliferate and produce sulfur in quantities considerably higher
than those, which carpeted the tube's inside walls at the beginning of the
experiment. Thus, the result is not a concentration but a creation.
The test tube (Erlenmayer type) is isolated by a cotton
cork, which works as a filter. (The air always contains some SO2, but the quantity is too small to be
able to start a culture; that is why this thiosulfate [hyposulfit] of sodium is
added.) The aerobic development of the thiobacilli shows that the latter need
the oxygen of the air. O is their
"raw material" for "producing" S. However, this example
shows that it is not always advisable to start with chemically pure products
purged of sulfur in an atmosphere without sulfur. It is untrue to say that the
experiment will be conclusive only if there is no sulfur in the beginning of
the operation; such an experiment – in this special case – would be likely to
fail. It is most important to know the sulfur weight at the beginning and end
of the operation. The difference between them reveals the endogenous production
by transmutation.
XIV CHLORINE
Chlorine probably had numerous origins.
Plants seem to be able to produce it. Branfield* gives a
quantitative analysis of the ashes of some plants and remarks that chloride is
found in rushes, water lilies, sphagnum moss, ryegrass and nettles, growing
where there is only fresh water.
[*Branfield, Continuous Creation, Routledge & Kegan Paul
Publ., London, 1950.]
Chlorine is a vital element of great importance. If life
originally sprang from the sea – where the salts are essentially chlorides –
if our blood plasma is essentially a liquid salted with sodium chloride, there
is a reason. Why is it not salted with sulfates, carbonates, nitrates, etc.;
why chlorine?
The chlorine content in an organism seems to be constant.
There are approximately 9 g/l of NaCl in the plasma. (Fish have organs, which
are able to eliminate the sodium chloride excess; the water contained in their
flesh is only salted at approximately 9-10 g/l.) This suggests that life began
in water having the same initial content of NaCl, thereby making Na and Cl
vital elements. There could not possibly be life without the proper proportions
of these two elements.
If the chlorine content varies only to a limited extent, it
is because chlorine is a regulator and a reversible element. It may appear from
nuclido-biological reactions, thus remaining constant and independent from
exterior sources. Let us recall a few reactions:
N2 =>
C + 0
Na => Li + 0
An organism contains Na and C, and
1123N + 612C =>
1735Cl
The relation between Na and Cl is obvious. Hence an organism
has elements enabling it to produce Cl endogenously.
Oxygen with interplanetary lithium originally produced sodium.
It is possible that sodium and chlorine could have appeared at that time.
Nitrogen, which is activated in various ways, produced oxygen and carbon. Let
us point out that the reactions cited above are isomers of the following ones:
Cl => C + Na
=> C
+ (Li + O)
=> C
+ O + Li
=> N2
+ Li
=> Si + Li
Nitrogen could thus have given silicon, and the latter combined
with Li produced Cl. Or, still another
representation of the same reaction:
N2 =>
C + O
↓ ↓
↓ O + Li => Na
C + Na => Cl
proving that Na and Cl could have originated simultaneously
from N2, with one atom of Na
for one of Cl. Hence, no compound other than NaCl is possible by way of
transmutation.
It seems that potassium can only be a subsequent creation
(as is the case in an organism). If K =>
Li + 2 0, then K ought to have been produced in two phases. It derives from Na
+ O (as in the organism), i.e. from (Li + O) + O. But if sodium chloride is present, one must expect an
oxydo-nucleonic addition giving NaCl with KCl.
Of course, if Na can take O it can also take H. In NaCl solutions potassium chloride will
be formed at the same time as (Na + H) => Mg.
Such is the case with the potash lies of the Stassfürt type, which are found
also in Alsace, France.
We cannot reject the possibility that fluorine is linked to
chlorine. We have:
Cl35 – O16
=> F19
Cl37 – O18
=> F19
or we can have the opposite. From the reactions N2 =>
C + O and Na => Li + O, one may
conclude that C and Li, produced by nitrogen and sodium, give:
C + Li => F
Since oxygen is available, one has F + O => Cl.
Furthermore, F + C =>
P, so that a phosphorus production occurs. The elements P and F are often together
(in bones, ores). It is easy to see that the whole system of Nature is
maintained by a constant balance of a few elements.
I shall point out many hypotheses accompanied by
cross-references. For the time being they are only open roads for a more complete
study.
XV MANGANESE AND IRON
The manganese-iron link was verified long ago by
agronomists. Plants require specific bacteria for absorption of manganese and
iron. However, curious phenomena have appeared to researchers: a manganese
excess produces the same effects as a lack of iron. In other words, an excess
of manganese impedes the assimilation of iron, and vice versa. Many of these
researchers have recently been pointing out a source of protons in these
phenomena. But they could not discover the process, which developed these
protons.
Besides these phenomena, which agronomists have been studying
for fifty years, a study is now being conducted on the use of the
above-mentioned bacteria to enrich manganese ores.
Another fact attracted my attention: manganese formations on
some cave walls and on the temples of Banteay Srei in Cambodia are struck with
a "black disease." The
surface of these stones becomes black and this black layer is 5% Mn, although
there is only 0.05% Mn in the stone. This increase in Mn did not seem to be due
to a simple migration, since the total manganese contained in the outer layer
was greater than that which the entire stone could contain. In my first book
(1960) I had already foreseen the possibility that iron and manganese are
separated by only a proton.
Fe56 – H1
=> Mn55
I asked the conservator of the monuments to find out if
there was iron in the stones. An analysis of the pink sandstone revealed 5 to
15% iron, depending on the stone's origin.
It then had to be ascertained that it was iron, which had become
manganese. Microorganisms (actinomycetes and bacteria) were set apart and a
culture on ferrous sulfate was started in a laboratory. Manganese was produced.
In 1963 after ten years of research, Professor Pierre
Baranger (chief of the laboratory of organic chemistry at l'Ecole Polytechnique
in Paris) verified that during germination manganese had disappeared while an
equal amount of iron appeared.
Professor Baranger made the following verification: in leguminous
seeds germinating in a soluble manganese salt added to water, a great part of
the manganese disappears while an equal amount of iron appears. The manganese
that disappears represents 25 times the manganese weight of the seed. Thus
nature works wonders; it works with a coefficient of security so that if the
seeds do not find good conditions for germination they have enough vigor in
their diastases to germinate anyway.
The Mn content in seeds is variable according to the seed's
species. In the buckwheat seed there are 30 mg of Mn per kg of fresh matter and
in a 45% sifted flour there are 7.5 mg/kg.
Manganese is found in the blood and in all the tissues; it
is a "giver" of oxygen. Its important role in biology, however, is based
on the fact that the peripheral electrons of the N layer are weakly joined,
largely because the sub-layer "d" of layer M is incomplete. Therefore
there are many valences and a facility in passing from one oxide to another.
Manganese is used often by doctors who have found that a
manganese deficiency causes some types of allergies. (It has been verified with
the spectroscope that in 50% of all allergies the plasma has suffered a loss of
Mn.) A prescription of 5 mg of Mn, taken twice a week for ten weeks, can
cure asthma, hay fever, and other intolerances.
Agronomists also widely use Mn to get rid of the bad effects
of sea salt in the soil. (50 kg per 2.5 acres make sowable dried-up fields,
which have been reclaimed from the sea.)
The biological role of manganese is certainly important in
man and animal, but it has not been studied enough. Its role is best known in
plants. Some plants cannot, at a certain stage of their development,
"fabricate" the necessary manganese and therefore must find it in the
soil. This manganese will not become assimilable until the manganic bacteria
proliferate.
The presence of Mn has been observed in some enzymes. There
is a certain oxidizing enzyme in the lacquer tree, which fixes the oxygen on a
latex alcohol. But this catalytic action of the enzyme is linked to the
presence of Mn. If Mn is missing, there
is a total inhibition of the enzyme. The absence of Mn, however, does not
indicate a deficiency of this element. The deficiency originates most of the
time from the difficulty the roots have in assimilating Mn when the soil has an
overly alkaline pH.
Some plants are able to produce the missing manganese, while
others (oats, etc.) are not. The same is true of animals at certain periods: a
small reduction of Mn in the diet of female rats causes them to stop nursing.
Male rats become sterile.
The human organism seems to contain enzymes, which allow
iron to change into manganese and vice versa.
A manganese deficiency comes from a pathological problem in the
production of a specific enzyme. When this is the case, an organic manganese
supplement becomes "necessary."
Iron is a vital element, which is indispensable to animals
and plants. It has been established that iron is also linked to the process of
photosynthesis.
Nature "chose" manganese to constitute the stock –
normally oxidized and stable – that is the purveyor of oxygen and metabolizes
the ferrous ion. (It seems that "the enzymes called oxydases owe their
property of fixing oxygen to their ever-present manganese," wrote G.
Bertrand.),
It is from such a reaction that Life borrows the necessary
iron.
One can understand now why variations of Mn content do not
have much effect on a healthy person. It is not as Mn that manganese comes
into play in chemical reactions. Rather, this happens after it has been
transformed into iron. The organism is sensitive to variations of Fe, not of
Mn!
Iron is present almost everywhere. Even in "pure
clay" (kaolin) there is 2% iron oxide. In other clays there is 10 to 20%,
and in bauxite 30%.
There is no need to look for iron's origin in the center of
our planet; it is a "surface formation" at the level of the earth's
crust, even in the case of the so-called "deep lies."
Lies found deep in the ground can be carbonates. How can this
be explained? There is neither CO2
from the air nor CO2 brought
by water. One can see from the reaction I observed what the process is: the
silicon of the primary rocks can, with Li, give Fe. But other nuclei of Si,
when under pressure, can become C + O. Hence we have the chemical elements for
the formation of the carbonates.
The oxygen in turn can give O + O => S, under conditions of pressure,
so that there are sulfurs (pyrites) in the depths hut oxides on the surface.
"On the surface, and often at a certain depth,
superficial alterations have transformed the carbonate into a pure hematite, a
formation difficult to explain since a mere ordinary and superficial
alteration should give limonite and not hematite," says F. Blondel.* He goes on to say, "The hematite
production on the surface is not well-clarified."
[*Chroniqe des Mines Coloniales, Sept. 1955.]
However, we have seen that there is oxide on the surface,
due to an endogenous production of oxygen. (Oxygen can also be liberated from
potassium to give lithium.)
At the level of the nucleon an abundance of Si, along with a
situation of stable saturation produced by 4.Li, results in an abundance of Fe.
There is 91.64% Fe56 and 5.81 % Fe54, since
around Si30
there is 4.Li6 instead of 4.Li7. The 2.21 % of Fe57 comes from 4.Li7 with Si29, whereas
0.34% of Fe58
comes from Si30.
Manganese, which has an odd number of protons, cannot have
an odd number of neutrons. There will then be an odd-even number so that the
number of neutrons will be superior to the number of protons by at least five
units. (This is also the number of neutrons plus protons in the preceding
element, vanadium.) The minimum then is 30 neutrons, which give 15 nucleons.
The following odd number is copper. Cu63 has five more neutrons than
protons. Manganese thus cannot have any more or less, than five neutrons in
conjunction with the number of its protons. That is why there is no isotope:
there is 100% Mn55.
From this fact it is clear that only the isotope 56 of iron
can give Mn by losing one proton.
Manganese and bacteria
For many years it appeared that the presence of manganese
was linked to the presence of microorganisms. Many studies have been made to
this effect, but here again research has been interpreted according to a
classical chemical reaction. The same phenomenon can be explained by biological
transmutations: the bacterium takes H from Fe in a medium poor in ions of
hydrogen (high pH – basic). There is thus an increase of Mn. On the other hand,
if there are too many H ions (low pH – acid medium) the bacterium, in order to
re-establish the equilibrium, takes H;
Mn disappears, becoming Fe.
Precise research had never been done to determine the source of Mn,
which was "fixed" by the bacteria, nor where the
"restituted" Mn went!
If Mn is "restituted," this seemingly means (in
terms of classical chemistry) that the solution gets richer in Mn to the detriment
of the bacteria. A systematic study of the influence of pH must be made again
using ferrous solution and having in mind not a simple exchange but a
transmutation. An exchange may occur, but that doesn't explain the appearance
of Mn in such great quantities. The correct explanation is transmutation, and
it was verified.
It seems that the bacterium envelops itself with a layer
rich in Mn. This phenomenon has an industrial application: mangano-bacteria in
a slightly basic solution, sprayed over finely-ground ores very poor in Mn,
cause a proliferation of these bacteria which, in their porous membrane of Mn,
are very easily separated when floating. The result is a concentration of Mn.
However, a microbiological study of Mn shows that there is
not only a bacterium involved. In the bottom of the Atlantic and Pacific oceans
nodules rich in manganese have been found and "fished" on a large
scale. These nodules have been studied biologically everywhere (in the U.S.A.
by Ehrlich, Mero, etc.*), but no one has thought to make a systematic
experiment without manganese, in ferrous solution. The given explanation is
that in the sea the nodules are formed from a bacterian action, which oxidizes
the manganese in order to allow it to fix itself on the nodule.
[*H. Erlich, "Bacterial Action on Manganese in Nodules
Enrichments," App. Microb., 1963, II-I, 15-19.
This is the first
photograph ever made of the transmutation of manganese into iron.
Fig.10 In a Petri
dish, associations of selected bacteria are cultured in mineral solutions fixed
by a gelose. A manganese powder is then sowed into the culture. After a certain
time, a rapid proliferation is established and the ferro-bacteria transmute the
manganese into iron. The iron oxide drag is visible here in this photograph
taken by L. Kervran.
Be that as it may, the general conditions for the
proliferation of the bacteria, which "fabricate" the manganese, are
now known. Associations with actinomycetes initiated the industrial studies
whose first application was the enrichment of ores poor in manganese but rich
in iron. The presence of the bacteria transmuting Fe into Mn is certain here.
One can increase their proliferation and activity considerably by controlling
the temperature and pH and by adding 0.1 % of peptone to the solution. (These microorganisms
are heterotrophic.)
To show how numerous are the mangano-bacteria (found in all
types of soil along with other microorganisms), here are some data for a soil
in which pH= 6.7, prepared for a culture of oats.
(Numerals refer to number of bacteria per gram of humid
earth.)
(in millions)
Diverse bacteria 225
Actinomycetes 4.8
Mushrooms 0.045
Mangano-bacteria 0.315
(Analysis by M. I.
Timonin)*
(The mangano-bacteria
were counted by the Gerretsen Method.)
[*See Bibliography]
Hence without sowing bacteria in a natural soil, one finds
315,000 mangano-bacteria per gram of earth; one may, at will, either annihilate
them or favor the activity.
The above data are not given here as an average. They may
vary greatly.
A soil (superficial layer) gave:
Total bacteria 564.2 million*
Actinomycetes 2.3 million
Mushrooms 0.0338 million
Denitrifying
organisms 0.6 million
Mangano bacteria 255.0 million
[*per gram of earth, thus 800 times more mangano-bacteria than
in the preceding example and twice as much total bacteria.]

Fig.11 Angkor-Tom: "black leprosy"
(manganese) invades the monuments.
Geology
The manganese link with iron is observed in its ores. One
often finds 15 to 20% Fe in ferruginous clays. The ores are classified in four
categories:
Manganese ores 40 to 63% Mn 0 to 10% Fe
Ferruginous ore of Mn 25 to 35% Mn 10 to 30% Fe
Iron ore containing Mn 5 to 20% Mn 30 to 40% Fe
Iron ore 0 to 5% Mn 45 to 70% Fe
For example, the Ouenza ore is an iron ore, which is 4% manganese.
It remains a hematite.
The Kizova ore (of Czechoslovakia) is a ferruginous ore of
Mn with 26.85 MnO and 32.56 FeO. It is an oligonite.
On the other hand, the Moanda ore, found near Franceville,
which contains 50% Mn, is an ore of manganese.
The Pre-Cambrian rock in this region (N.E. of the Gabon, 500
km east of Libreville) contains an important lie of iron ore. This shows that
Fe and Mn can have the same rock of origin but that the change from Fe to Mn probably
occurs from causes other than biological ones.
It is clear that Mn and Fe combine well. The microorganisms,
which initiate this liaison, have been sampled previously and the production of
Mn from Fe in the laboratory has been accomplished successfully. Private
laboratories are even determining in which economical conditions such an
operation can be launched industrially.
XVI VARIATIONS OF MINERALS IN DRIED FRUITS
Many scientists now see the impossibility of explaining the
mineral variations in dried fruits by means of chemistry alone. I cannot cite
here all that has been brought to my attention concerning this subject.
Notwithstanding, I shall mention an article by H. C. Geffroy, which appeared in
the review La Vie Claire (December 1966) and gives an analytical comparison of
a fresh almond and a dried one. According to Lucie Randoin, the article points
out; there is 87% water in a fresh almond and only 4.4% water in a dried one.
Here are some of the results Geffroy gives of Miss Randoin's analysis (for 100
grams of almonds):
– Nitrogenous
matter
5.67 g in the fresh almonds
18.10 g in the dried almonds
– Fat
2.19 g in the fresh fruit
54.20 g in the dried fruit
– Mineral salts
0.96 g in the fresh fruit
2.50 g in the dried fruit
It is obviously impossible to support the classical
explanation according to which the difference in composition is due to desiccation,
for in that case there would be an equivalent concentration of all components.
In another review, "A Table" (April 1967), Mr.
Geffroy draws attention to a comparative chemical analysis of the fresh plum
versus the dried. The results are slightly different from those cited by Lucie
Randoin, but these minor differences might have been due to the type of soil
used.
Since Miss Randoin's figures are more detailed and since she
specifies different kinds of fruit, we shall use her results.
The dried prune, when analyzed, has 2.4 grams of water (per
100 g) less than the fresh plum. But the carbohydrates are seven times more
concentrated in the dried prune. Desiccation alone would increase the organic
and mineral components 6.2 times. The lipids and proteins increase only three
times. It is as though they diminished relatively, and it is probable that
molecular transformations of a biochemical order changed these lipids and
proteins into carbohydrates.
Minerals increased only three times; sodium, 3.3 times;
potassium, 3.8 times; magnesium, 4.4 times; phosphorus, 5 times. On the other
hand, sulfur, which increased 6.1 times, kept its relative rate of increase
unaltered. Iron multiplied by 7.2; copper by only 1.6. Manganese diminished
greatly: there was 9.17 times less manganese than if there had been only
desiccation.
From this we can see how important it is for dieticians to
know the respective variations and to be aware of the difference between
taking fresh and dried fruit. Let us take some results given in the "Table
of Composition of Food" by Lucie Randoin (from the Academy of Medicine). The
calculations pertain to 100 grams of seeds.
– in dried soya
beans, 580 mg of P
– in germinated
soya beans, 67 mg of P
– in dried soya
beans, 280 mg of Ca
– in germinated
soya beans, 48 mg of Ca
Where did the phosphorus disappear to once the soya beans
had germinated? (There was 8.6 times less.) Where did the calcium go? (There
was 5.8 times less.)
Let us take simpler cases where desiccation does not enter
into play (at least in principle; we shall see later that there is something
else).
In the green pea there is:
S P Mg
Ca
when green 60 122 42 26
when dried 219 380 130 60
These abnormalities did not surprise anyone. If there had
been merely desiccation (loss of water), the ratio between the minerals would
have remained the same.
Let us take the case of the banana:
S
P Mg Ca
fresh 12 28 35 11
dried 36 90 105 21
We can see that in the drying process Sand Mg become three
times more concentrated while Ca multiplies less than twice and P less than 3.2
times.
The P /Ca ratio is:
2.5 in the dried
fruit
4.3 in the fresh
fruit
For the fig:
S P
Mg Ca
fresh 10
30 21 32
dried 34 116 72 170
The dried/fresh ratio is 3.4 for S and for Mg. On the other
hand, it is 5.3 for Ca and 3.8 for P. There is a slight increase in P, but a large
increase in Ca.
In grapes, the phosphorus content changes from 20 to 145; Mg
changes from 10 to 36, Ca from 20 to 40. Although P increases 7.2 times, Ca
only doubles while Mg increases 3.6 times and sodium 11 times.
Variations of oligo elements in dried fruits
Here are some results, according to L. Randoin, of a few
cases of variations in oligo elements:
In the dried chestnut, iron is multiplied by 2.3 and copper
by 1.08, so that Fe/Cu = 1.33 in the fresh chestnut and 2.86 in the dried – a
117% increase. These findings seem to indicate that some copper became iron.
However, for the fig we have:
Fe/Cu = 1.50/0.06
= 25 in the fresh fruit
3.0/0.35 = 8.57 in the dried fruit
Hence Fe/Cu is 191 % higher in the fresh chestnut than in the
dried. Iron only doubles, while the water content is 3.4 times less. On the
other hand, copper increases 5.8 times. Thus, some iron disappeared and the
amount of copper increased.
In the peach the concentration of mineral elements is five
times greater; the water content is 3.58 times less. But it is not the same for
the oligo elements.
The Fe/Cu ratio is:
0.40/0.05 = 8 in
the fresh fruit
4.0/0.26 = 11.1 in
the dried fruit
There is thus ten times more iron and five times more
copper, indicating that the very noticeable variation of Fe/Cu does not derive
from Cu and that iron has another origin.
In the pear there is a great
variation of Fe/Mn:
0.40/0.06 = 6.6 in
the fresh fruit
1.80/0.20 = 9.0 in
the dried fruit
meaning an Fe/Mn increase of 36%.
For the apple it is the opposite:
Fe/Cu = 0.40/0.10
= 4 in the fresh fruit
1.44/0.58 = 2.48
in the dried fruit
For Fe/Mn we have:
0.40/0.04 = 10 in
the fresh fruit
1.44/0.20 = 7.2 in
the dried fruit
Thus there is a different enzymatic behavior in the pear
than in the apple. In both, Fe/Cu = 0.40/0.10 = 4 in the fresh fruit, whereas
Fe/Cu = 9 in the dried pear and 2.48 in the dried apple, due to the formation
of copper in the dried apple.
XVII AN INTERPRETATION OF AN ANALYSIS MADE ON
RYE-GRASS
The interpretations of this analysis by the Laboratory of
the Société des Agriculteurs de France* were included in the same sheet in
order to gather together all the numerical values necessary for the
understanding of the results.
[*Officially recognized by the French Ministry of
Agriculture for arbitration and expertise in cases of litigation regarding
fertilizer analysis.]
Dosages of magnesium, calcium, and copper were given to
1,000 seeds of rye grass from Italy (Rina Variety). These seeds were not
germinated but were kept as controls (column 5).
A similar lot (in two Petri dishes, 500 seeds per dish) was
germinated for 29 days on porous ashless paper soaked with Evian water (column
6). At the end of the experiment, the remaining water was measured (430 cm3 were used for the entire culture). The
Mg, K and Ca contents of the Evian water are indicated in column 7.
The seeds were germinated under a plastic sheet in order to
avoid any introduction of dust. These seeds were similar in weight to the ones
being kept for later comparison (column 5). The composition of the Evian water
is in column 7. The total of the elements introduced in the Petri dishes is in
column 8.
With no supplement for the seeds other than water, it was
found that the harvested plants had a different content of Mg, K and Ca (column
6). The differences between (6) and (8) are shown in column 9. These
differences are expressed in percentages in column 10.
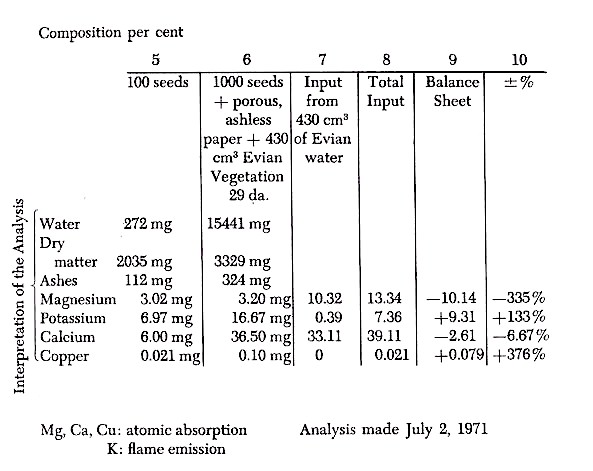
Interpretation
There were 3.02 mg of magnesium in the seeds. With the addition
of water there should have been 13.34 mg, however there were only 3.20 mg.
There was a 10.14 mg "loss," i.e. 335%.
For the potassium there was a 9.31 mg increase, i.e. 133%
more.
The calcium did not decrease much; but the copper increased
by 376%.
An important lesson
Mg diminishes by 10.14 mg, while K increases by 9.31
mg. Notwithstanding, we could not
conclude from this experiment alone that when K increases, Mg decreases (for
although they compensate each other on the ponderal plane, the difference in
nucleons on the atomic plane corresponds to the nitrogen content, which was
not measured).
Although the reaction would be valuable for the various
stable isotopes of Mg and N, as well as K, we will not write 12Mg + 7N
:=: 19K without conducting research on the intermediary N.
This is because there is another possible reaction in the mitochondria: Mg giving Ca by nuclear addition of O. The excess Ca which cannot escape makes the
pH rise, thus triggering the transmutation of Ca into K by taking away an H
proton from Ca. K remains and can
escape through the membrane while H, now liberated, makes the pH of the
mitochondria diminish. Thus it is clear that the subtraction K – Mg, for example,
does not necessarily indicate the intermediary. Nature mostly operates by
transmutations with ± H or ± O.
But whatever the intermediate mechanisms, one fact is verified: Mg decreases
and K increases, in this specific experiment. (One should be careful not to
generalize, because each plant behaves differently.)
This research was initiated in the hope of confirming
previous work by verifying precisely that Ca does not vary in the cultivation
of a plant that is unable to "make" the calcium indispensable to its
metabolism. That is why the culture was made with Evian water, which is rich in
calcium.
The calcium absorption by the rye grass is remarkable since
the water provided 33.11 mg and 36.50 mg were found in the plants (for lots of
1,000 seeds). This means that all the calcium that was fixed in the plant,
including what the seed contained, came from the water. This process is
characteristic of plants unable to make their own calcium. Experiments of the
same kind in waters either rich or poor in calcium reveal the differing behavior
of plants able to manufacture their own calcium.
Remarks
The behavior of magnesium is interesting to observe. One can
see that the seeds contain 3.02 mg of Mg; while after germination the plants
contain only 3.20 mg of Mg. This shows that germinations made with distilled
water do not permit precise study of the biological transmutation phenomenon:
it was necessary to supply 10.32 mg of Mg with the water to verify that in the
culture a great quantity of Mg disappeared (a disappearance of approximately
three times the amount of Mg contained in the seed). An experiment involving
several lots totaling 21,000 seeds (50 grams) of rye grass showed that the Mg
variation is slightly noticeable in dematerialized water. The same series of
experiments also showed a very small variation of K, which is normal since in
this case one is only "gambling" on the reserves of the seed. But
here, with a small amount of K being provided by the water, the total potassium
is more than doubled, whereas there is a consumption of the magnesium that was
provided.
Conclusion
The mineral elements given to the soil should not be those
taken away with the harvest; their choice should depend on what was transmuted
by the plant to give what was gathered at the harvest. The latter is what we
call "substitution," whereas classical agronomy arrived at the sad
policy of unbalancing soil and plants with the erroneous
"restitution" technique.
Our case is made manifest by the behavior of Mg. If we limit
ourselves to an analysis of seeds and plants, we might think that there is
little change in Mg content and say, along with those who practice restitution,
that bringing Mg to the soil is useless. But we see that it is important to
bring Mg that the plant uses this element after transforming it. On the
contrary it is useless, despite appearances, to provide K if the plant can
obtain Mg, since the plant produces K. Besides, several experiments have shown
that more K is found in the plant than what was originally given to the soil.
(A frequent origin of K is Ca – H.) The whole classical technique of mineral
fertilization must be reevaluated, especially in schools of agriculture.
XVIII TRANSMUTATION OF RADIOACTIVE WASTES
Do the reactions, which I propose, have a possible
application for the destruction of radioactive wastes?
At first glance one might remark that my reactions have not
been observed in the presence of radioactive phenomena. But one should not
dismiss a possible application a priori, without studying it, and I have done
no research on radioactive substances.
An objection presents itself: since these very
transmutations, which were studied and applied, are happening at a biological
level, it is unthinkable to anticipate that microorganisms could transmute
radioactive wastes into stable nuclids.
The destructive effect of the radioactive radiance is being
utilized to kill cells and to sterilize various products. But nothing is
absolute! Not even this law of the biological effects of radiation! A bacterium, which can support ten million
roentgens for eight hours, has been discovered! The mortal dose for man is 500 roentgens. This bacterium, of
the Pseudomonas type, was discovered in 1958 in Los Alamos, U.S.A., in the
water of a nuclear reactor. It liked the medium so much that it reproduced
every twenty minutes.
A little later in Lucas Heights, Australia, a verification
of the same kind was made. In stored heavy water there were two million
bacteria per cm3. Only 1,000
of these bacteria were left when the heavy water came out of the reactor. The
content rose to 10,000 at the exit of the ion exchanger. These bacteria were of
the "Pseudomonas, Bacillus", and "Achromobacter" types. It
can be seen that these bacteria can multiply in heavy water, which is not
conducive to the life of most organisms.
However, we can see from the above that some bacteria can be
cultured very readily on radioactive substances. It would be wise to find out
both if these bacteria can help make the transmutations, and which ones they
can make. Transmutations can also be made by mushrooms, algae, and enzymes.
Here is a theoretical example: if strontium 90 could be
"fritted" with fluorine, it would give non-radioactive silver (Sr90 + F19
=> Ag109),
providing that the fluorine could enter in this manner in a nuclido-biological
reaction.
Studies should be made to find out if C14 and K40
enter into the reactions of transmutations. It is probable in the case of K40, for noticeable variations of this
nuclid are found in the potato, for example. One thing is true: it is almost
stable nuclid. If formal proof were given that it did enter in the
nuclido-biological reactions to give a stable or short-lived nuclid, however,
this would constitute a breach with the actual theory of radioactivity.
A study* was carried out by L. Magos, Tuffrey, and T. W.
Clarkson (Research Council Laboratories of Carshalton, England), who used
grindings of rat kidneys homogenized with adduction of HgCl2 (radioactive isotope 203Hg). Samples were tested at the Geiger
counter at staggered times. The period of this isotope is 46 days. However, the
diminution of activity corresponding to the disintegration of this radioactive
isotope was much stronger than anticipated.
[*"Volatilization of Mercury by Bacteria," British
Journal of Industrial Medicine, October 1964, pp. 294-98.]
Where did the mercury go? Since it disappeared, everyone
believed that it had "evaporated." But here again, isn't this an
unverified assertion, deduced from the sole fact that mercury
"volatilized"?
Meanwhile, it became apparent during the experimentation
that mercury does not "volatilize" regularly. For according to the
law of diminution of radioactive activity, there should have been a regular
curve, exponential and well known. However, a counting made sixteen hours after
the first verification showed clearly that there was a diminution of mercury,
conforming to the law of radioactivity. But after 32 hours there was an unexpected
diminution of mercury. After 48 hours the difference took on great proportions.
The merit of these researchers was in thinking that the phenomenon
could be produced by a microbian action, that the latent period (which lasted
at least sixteen hours) could correspond to the incubation period of a
bacterial colony. Cannot the effect of toluene (methylbenzene) be explained by
the fact that it kills the bacteria? However, this hypothesis was a priori
hazardous because it was believed that bacteria do not attack heavy metals, the
latter resisting all actions of biological products.
Penicillin kills bacteria, and mercury disappearance follows
the same law, as does a sterilized solution in the autoclave. There is 50% more
mercury lost in 48 hours in a contaminated medium. This event is not imputable
to the disintegration of the radioactive isotope. If one takes a sterile sample
and inoculates it with only 1.10 – 5M
of HgCl2, which has already
started to lose its mercury, one verifies that there is no latent period. The
proliferation is immediate and in 24 hours 60% of the initial activity subsides,
whereas a non-inoculated sample kept for later comparison loses only 2 %.
The identification of the most active bacteria revealed a
"Klebsiella aerogenes", a bacterium of the "Proteus type",
an active microorganism, which could not be identified, and a great number of
other microorganisms carrying on little or no activity at all. In the city
water used for the experiment was found the "Pseudomonas pyocyanea",
which is very active. A "Diplococcus" was also found, but it does not
die from toluene; it only becomes partially inhibited.
Unfortunately, researchers considered nothing but the disappearance
of mercury by volatilization. Nevertheless, they verified their theory. They
declared the phenomenon to be a natural one, basing their hypothesis on the
diminution of activity measured with the Geiger counter. Thus, haven't
undisputed results received a weak interpretation, lacking essential analysis
and measurement?
The researchers did not suspect that the disappearance was
due to the biological transmutation of mercury. Evaporation is negligible a
priori; mercury boils at 360° C and its vapor tension is very low (40 to 50°
C). But the experiments were conducted at the ambient temperature of 20°
C! This fact should have alerted the
researchers and could have prevented them from adopting the postulate of
evaporation.
This experiment leads us to qualify what we said about radioactive
elements: it was not dear to us whether superior organisms (animal and
vegetal) performed biological transmutations on radioactive elements. However,
the behavior of bacteria is always full of surprises. Regarding the
transmutation of iron into copper, I mentioned in a previous book* that some
bacteria proliferate in pure sulfuric acid. I also cited some Pseudomonas,
which live in the heart of an atomic reactor in heavy water and receive more
than 1,000 times the mortal dose for human tissue. The same is true with the
"Micrococcus radiodurans", which resists 3,000 times the mortal dose
for a mammal. During the experiment on mercury it was also found that there is
a Pseudomonas species, which "digests" radioactive mercury, making of
it another element unidentified to this day. But what happened to the excess
neutrons in the radioactive nucleus? Since there is a decrease in
radioactivity, there should be no transmutation into another radioactive
element. Might a "conversion" of neutrons into protons possibly have
occurred? Only what remains at the end of the experiment will allow the pronouncing
of a verdict.
[*Transmutation à Faible Energie (2nd edit.), Maloine Pub.,
1972, p. 179.]
The perturbations caused by "Υ rays" impede
the reproduction of ADN. There is a progressive death of the cells. However,
the speed of reproduction is generally considerable in a bacterium, which means
that the bacterium's enzymatic activity is considerable. This explains how
bacteria can resist an irradiation several thousand times greater than the dose
that vegetal or animal matter can take.
Have we not here an open field where research concerning the
elimination of radioactive wastes can be conducted? I indicated this as a hypothesis in 1960. Don't experiments of the type cited here
seem perhaps to demonstrate that it is indeed happening? If this possibility were to be established,
it would be a great event since we would then be able to study the destruction
of the radioactive wastes, which pose such a threat to humanity.
XIX HOW TO MAKE EXPERIMENTS
WITH BIOLOGICAL
TRANSMUTATIONS SUCCESSFUL
In order to succeed in transmuting elements biologically it
is necessary to abandon certain concepts of the so-called "exact"
sciences, which are exact only for simple and isolated cases foreign to
biology. As I have already stated, biology is too complex a science to be
compared with these "exact" sciences. It is comprised of too many
interdependent parameters to be completely accessible nowadays – even with the
help of computers – by scientific methods based upon hypotheses, which are
solely mechanical. I must therefore reject the groundless assertion that all
natural phenomena are reducible to the phenomena of calculations. Anyone
subscribing to this idea will have an increasingly hard time proving his point
as more and more of life is revealed to us.
In the following review of a few general principles it is important
to beware of committing the opposite error by extrapolating certain
transmutations and saying to oneself, "Since Nature can transform one
element into another, it will be enough to have only one element at our
disposal and Nature will take care of the rest!"
The transmutations are operations requiring a specific
production of enzymes and a medium allowing the physiological development of
cells (or microorganisms). One kind of plant will thus make a transmutation
that another cannot make. Or else, a transmutation will be made in one
direction in a growing plant, whereas it will be made in the opposite direction
in a germinating seed of the same species. The same is true with animals: a
reaction will take place in one direction under a certain set of conditions,
and again in the opposite direction, with the same animal, when different
interior and exterior conditions are met. On the other hand transmutation may
take place in no direction at all, regardless of conditions, thereby requiring
the animal to obtain a specific element from outside. Generalizations in
biology reveal an ignorance of life's complexity.
A transmutation is not always absolute. For example, it
is often impossible for a plant, seed, or animal cell to make the transmutation
into a specific element if this element is not already present to serve as a
catalyst. This principle should be kept in mind. Let us recall Jacob,
Lwoff, and Monod, Nobel Prize winners who showed that for a gene to be active
requires the "influence" of a certain element which "releases
the brakes" and prevents the synthesis of the enzyme.
That is why it is usually vain to try to produce an element
with biological transmutation if that element is not already present. In other
words, what should be sought is the increase of an element (which always leads
to the diminution of another), not its appearance from zero.
One should never start with absolutely pure products. The
metabolic conditions of the animal or vegetal cell must be retained in order
to verify an increase of the elements, a proof that will have no room for
contention on the part of those chemists who are prone to reject everything new
a priori.
Generalities
Conditions for transmutation
Many kinds of seeds have been germinated in my
experiments: Watercress, lettuce,
parsley, etc. The leguminous plants most often used were fava beans, soya
beans, etc. For practical reasons, however, lentils and vetch were most often
elected. Their size is uniform and they are easy to calibrate and not too
large, thus eliminating the possibility of irregular germination. Poor germination
of one seed can cause an appreciable error in calculations. Doubly-distilled
water should not be used. Again, a chemical preparation designed to satisfy the
rigorous conditions of "exact" sciences would lead to excessive
simplification since, biology being complex, there is no life without several
interactions. The term biochemistry itself means that the biological precedes
the chemical.
In other words, I consider it an error in such experiments
to use an absolutely pure culture medium in studying the variation of an
element. One should proceed with a complex medium, which is close to the
natural medium but missing in the element to be studied. The results will then
be quite different. In the former case there are too many deficiencies and poor
metabolism; the transmutations are of 1 to 3% only. Thus arguments arise. In
the second case the variations are always over 10% depending on the element,
often 20 to 30% (sometimes ten times more) for plants, and 30 to 300% for some
animals.
Nevertheless it is sometimes impossible to completely
exclude a certain element at the start, so as to measure its exact production
after the transmutation process. Experience shows that there are cases where,
for certain cultures, some elements are indispensable at the start. Proliferation
will fail to take place if the amount of these elements is too small.
Microorganisms
That is why I am not in favor of the sterilization of seeds,
soil, etc. I have heard that precise research can be made only on sterilized
seeds in a sterilized liquid or solid medium. If the microorganisms are
excluded, however, the results will be whimsical. This omission would be a
confession that the activity of the microorganisms disturbs chemical research,
that the purpose of the research is not really clear.
Studies have revealed that various enzymes (the permeases)
allow biological membranes to receive certain molecules but not others. It
seems that there are perennial permeases, which are constantly being
synthesized, while others are synthesized only in the presence of the molecule
to which they must obtain entrance. Hence the results from a sterilized medium
are totally different from those obtained in the presence of microorganisms.
If one wishes to germinate seeds in a vat, it is necessary
that the plants grow in optimum conditions for vegetation; the earth must be
alive, i.e. rich in microorganisms. This earth should be well mixed and
homogenized. An aliquot portion should be set apart for use in measuring the
element on which the balance sheet is to be established. At the end of the
experiment the plant and soil must again be analyzed.
The water
The water used should preferably be source water. It is inadvisable
to use distilled or doubly-distilled water, for then the pH must be readjusted
by the addition of a calcium salt to place it between 7.3 and 7.5.
The best by far is natural, chemically untreated water. I
recommend Evian water, which is quite good for research on phosphorus since it does
not contain that element at all. In this case it is unnecessary to add a
calcium salt. Evian water contains it naturally, making its pH favorable: pH =
7.25. Here is what an analysis of Evian water gave (limited to those ions that
may interest us):
Ca++
= 0.07715;
Mg++
= 0.2432;
SO4– – = 0.0096;
Na+
= 0.00552;
K+
= 0.0089;
Fe++
= 0.00001;
NO3– = 0.00254;
No measurable trace of P or Mn;
Silica (SiO2) = 0.011.
The figures are all in grams per liter.
Phosphorus
variation
Preparation of seeds
As I explained, it is preferable to use calibrated lentils
or vetch. A small pack of 125 grams will do. One should be careful to purchase
lentils from the last harvest. Some are too old and therefore too dry. In
order to secure the success of the experiment one should verify in advance the
germination power of the lentils by trying them (100 lentils in a Petri dish).
99% of the seeds should be perfectly germinated. Any kind of water can be used
for this first try (faucet water, for example). The seeds should be hand
selected to eliminate those that have changed color or deteriorated in the
slightest degree (for example, those whose color is too brown as compared to
the green lentils). The lentils should be divided into lots, and for this there
are two options:
– Prepare lots of ten grams each, weighed to the centigram,
and write down the weight of each lot, which will be put in a crystallizing
dish.
– Or, do not weigh the lentils at all, and use 100 of them,
for example. A lot of ten grams of lentils contains approximately 130 to 140
lentils. A lot of 100 lentils weighs approximately 7.5 grams; the average
weight of one lentil is between 70 and 80 mg. It is 30 to 40 mg for vetch.
The advantage of counting the seeds is that there will be an
identical number of young plants in each crystallizing dish; while with the
other method there may be ± one seed. It is easier to maneuver with the
counting method, since a defective seed, unnoticed during selection, can be
replaced. For an experiment to determine P variation, after putting aside five
lots of 100 seeds to serve as a control, place the rest of the seeds in a basin
and cover them with Evian water.* They
should be left there 24 hours. They should be rinsed in clean Evian water in
order to remove all traces of dust, and because the 24 hour stay in the water
causes a visible darkening of the defective seeds. (At this point the seeds
have begun to rot and will not germinate well. They will contaminate the
others, probably causing inaccurate results.) Plunging the seeds in Evian
water does not provide them any phosphorus; hence there will be no
repercussion in the analysis. Thus, one should take 100 seeds from each
crystallizing dish and place them – side by side without touching – on a double
layer of porous ashless paper (ten lots of 100 seeds = 75 grams).
[*This only works for research on P variation (because Evian
water contains no phosphorus). The technique of washing with Evian water is not
to be applied in research on Ca, Mg, and K.]
The germination
We should not forget that what is sought is the phosphorus
variation. The ten crystallizing dishes containing the seeds receive a thin
layer of Evian water, which saturates the double-layered porous paper on which
the seeds are placed. These receptacles are placed in a greenhouse or in a
light room near the window so that they may receive good light while
maintaining a temperature of 22 to 25° C. The receptacles should be arranged in
two rows of five each or in one line. It is most important to place them in
such a way that they will receive a maximum of light. For every receptacle
there should be a corked bottle full of Evian water (100 to 125 cm3) with a
tube through the cork; the tubes' extremities are placed in the center of each
crystallizing dish, touching the porous paper. These bottles are placed
upside-down so that the porous paper is kept constantly wetted with water. The
humidity of the room must be maintained between 55 and 60%. There will be no
need to fill up the bottles a second time.
After fifteen days there will already be appreciable
results, but they will be variable from one experiment to another. In Germany,
Hauschka published results obtained from several germinations of watercress.
These germinations were made every fourteen days for a year. Hauschka started
one lot at the new moon, another at the full moon, and so on. He gives diagrams
of variations for K2O and P2O5, K and P varying in opposite directions with
a 16% average, and both changing directions depending on the position of
the moon. In watercress the phosphorus decreases if the germination starts
the day of the full moon. Hauschka's
diagrams* also indicate an influence of the solar cycle. Studies concerning
cosmic influence are now being conducted in several countries.
[*The Nature of Substance, ed. Vincent Stuart (London
1966).]
Analysis
The germination should be terminated two weeks after the beginning
of the experiment (which was started at the full moon) by putting the
crystallizing dishes in a drying stove at 60° C. They should be left there 48
hours, but if need be they can be left only 24 hours with the thermostat set at
90 or 100° C.
Right after cooling, grind the young plants in a mortar. Or,
use the sulfo-nitric acid method.
This destruction can be done in different ways. In general,
every laboratory chief has his own technique. Nevertheless, I would like to
point out that the acid being used for the destruction acts on matter not yet
incinerated which still contains some water.
The pH of the acid will thus rise, and it will be necessary
to supply more acid in order to maintain the pH at 4.8, an optimum level for
the extraction of all phosphorus.
Notwithstanding, the method using sulfo-nitric acid is
preferable (100 cm3 HN03 + 10 cm3 H2SO4). The mineralization is done as it is
taught to laboratory assistants: heating, sudden discharge of nitrous vapors,
cooling. The black and thick liquid thus obtained is slowly heated by adding 80
cm3 of a mixture of 20 cm3 of H2S04 in 1,000 cm3 of HNO3
until discoloration. Add 25 cm3
of doubly-distilled water and heat again until the nitrous vapors disappear.
When it is cool, strain the liquid from which the aliquot parts will be taken
for the dosage of the element.
The lots of seeds which were not germinated and which were
being kept for eventual comparison go through the same process: desiccation at
100° C, grinding, destruction with sulfo-nitric acid, analysis, etc.
Analysis must be done on the sulfo-nitric mother solution
(on an aliquot part 25 cm3).
The amount of phosphorus is determined by adding a sulfo-molybdic solution
(classical method). The phosphorus content will show a very significant
decrease, but further investigations are necessary because the process is not
the same in all plants.
Hence, when oats are germinated in water very poor in limestone
and absolutely free of phosphorus, there is a phosphorus increase in the
germinated plants after six weeks.
One may conduct research on manganese and iron variation in a
similar way. In fact, there will be an Mn decrease and a corresponding
increase of Fe in the germinating seed. (Let us recall that there is one more
proton in 2656Fe than in 2555Mn.)
Some plants, such as rye grass, make Mn, while others, such
as oats, cannot. In some plants Mn decreases.
It seems to me preferable to conduct this specific
experiment in an autonomous way, i.e. not using an aliquot part from the
preceding experiment relative to phosphorus. In fact, the experiment can be
made even more dramatic by adding to the Evian water (which does not contain
any) 10 mg of MnS04 · 7H2O
for 10 cm3 of water (2 mg of
Mn), which is approximately ten times more Mn than one lot of 10 grams of seeds
would contain. (This is true for plants, which cannot make their own Mn.)
The experiment is conducted in the same manner as the preceding
one, including the destruction with sulfo-nitric acid. Mn and Fe are then
weighed by the usual methods.
Analysis will verify that there is much more Fe after
germination, whereas Mn could have completely disappeared. This experiment
indicates the power of the enzyme responsible for this transmutation of Mn into
Fe.
Research on Mg and Ca variation
Using the preceding methods one may study the variation of
Mg and Ca in germination. If Evian water is used, it is necessary to measure
the quantity of water used and its Mg and Ca content.
A precaution should be taken, though: when the seeds are
soaked in water one day before the germination (to eliminate the deteriorated
seeds) distilled or doubly-distilled water should be used. Evian water would
make it impossible to determine the quantity of Mg and Ca absorbed by the
seeds.
The analysis of Mg and Ca requires some precautions. In fact
there are interferences of their ions, which are divalent, and there is a risk
of possible substitution in many reactions.
Specialists in analysis are well aware of this and know
which method to use when these two elements are simultaneously present. Here
are some figures concerning the content of these elements in fresh seeds: 60 to
140 mg of Ca in leguminous plants, for 100 grams of seeds; 80 to 200 mg of Mg,
depending on the species, variety, and soil. The different research conducted
on Mg variation during germination has shown a 9 to 16% decrease.
Complementary research
Potassium variations
Many experiments with microorganisms have shown an increase
in potassium when N and Mg are provided. This indicates the reaction 7N + 12Mg
:= 19K.
With the bacteria of saltpeter there is an increase of K and
a decrease of Ca. This is the reaction 20Ca
– 1H :=: 19K.
It is possible to note the K variation in germinating seeds:
1) Lots of 20 grams of watercress seeds are analyzed
to make a dosage of K. Other lots, germinated in distilled water without the
addition of salt, render 0.505 g of K2S04 per lot.
If magnesium nitrate is added to the distilled water, there
will be 0.570 grams of K2S04 (an average for three lots), which means
0.065 grams more than with pure water, or 11.4% more due to the simultaneous
presence of N and Mg.
The variations of K are complex, for they can produce Ca and
vice versa. They may also come from Na
(11Na + 8O :=:
19K). The K increase will be quite pronounced if
the germinating process is begun during the full moon and terminated at the new
moon. The result will be the opposite of the P variation (experiment conducted
by Hauschka*). The variation is greater for most seeds at the end of autumn.
[*The Nature of Substance.]
Note: Studies on potassium are delicate because there
could possibly be an exchange between the potassium contained in the glass and
the solution in the receptacle. An exchange is also possible during
manipulation, heating, or when the solution contains an ion monovalent like
ammonium. In other cases (for receptacles made of Pyrex, quartz, plastic, or
metal) the exchange is negligible, but it is safer to make a preliminary
experiment since some plastics give a potassium salt in the polymerization, and
this salt is never totally eliminated.
2) The link between Ca and K can be verified in the
following: in lots of watercress seeds (20 g each) 0.18925 grams of CaSO4 was found. The germination was done in
distilled water in which 0.200 grams of K2CO3 was added to each lot. After the
germination there was 0.22175 grams of CaSO4.
Ca increase: 0.0325 grams, i.e. 11.88% more Ca (average on
four experiments).
Conversely, if a calcium salt is added to the water, K
increases. In distilled water, with no salt added, 0.505 grams of K2SO4
was found for every lot of 20 grams. When calcium nitrate was added the average
increase was 0.0725 g, i.e. 14.37%.
XX AGRICULTURE
Classical agriculture (by this I mean the science that is
taught in schools) is a huge imposture, which is frightening in its cynicism.
It recommends the use of complete fertilizers, the word "complete"
being identified with N, P and K, as if these three elements were the only ones
to be found in plants. Its basic postulate is to restore in quality and
quantity the soil's elements taken from it by the yearly harvest. One may say
that this agriculture has enclosed itself in a dilemma:
a) If the law of restitution is to be satisfied (whereby one
implicitly recognizes that elements other than N, P, and K are taken away at
harvest), the plant would have to find these other elements in the soil in
limitless amounts.
b) Or, plants themselves must make these elements found at harvest
and not provided for the soil. Implicit in this alternative is a transmutation
of elements through a biological channel.
Otherwise, what kind of explanation can be given for the continual
removal of sulfur from the harvest, for example? Let us take a crop of oats and
circumscribe our study to a yield of 4,000 kg per hectare (1 hectare = 2.471
acres). In the grain alone (without taking into account what is in the straw)
there are 8 kg of sulfur per hectare. Where does this sulfur come from in impermeable
and clayey soils, as in the case of oats?
It is not a question of migration, for from where would
sulfur migrate? The same question can be posed regarding other elements. With
zinc, for example, we find 120 grams for 4,000 kg per hectare of oats. There
are also 160 grams of manganese per hectare.
After a few centuries of cultivation this adds up to many
kilos.
Not all these elements, however, exist in the soil in such
quantities. Plants find what they need, depending, of course, on the plant and
on the soil. One should not generalize. It must be established whether or not
the soil contains elements that the plant will utilize to fix other elements
not found in the soil, which are necessary to its growth. This being
established, all conditions must allow the transmutations to take place. The
latter usually occur in the presence of microorganisms for which the rhizome
must provide the conditions for a vigorous proliferation. Thus we must have the
synthesis of a biological agriculture, which is far-removed from classical
agriculture, concerned only with providing N, P, and K.
Application of biological transmutations
Biological transmutation explains the basic process of
biological agriculture, whatever the specific method. The biodynamic method,
as well as other methods involving active processes, uses decoctions (fermented
solutions of some plants very rich in various oligo-elements) to balance the
acidity of the soil. The oligo-elements are indispensable to the enzymes; the
enzymes (whose work is somehow activated by the activity of certain vegetals
and even mammals) are in turn responsible for the biological transmutations.
The activation of this enzymatic action by the plants also
explains the increasing success of phytotherapy and aromatherapy. The attempt
to provide plants with even a small amount of elements saturates them with
these elements and creates an imbalance in them and in the soil. There arises
a deficiency in other elements, and certain natural reactions are impeded.
Everything becomes fragile, the soil's health deteriorates, and the reactions
of the vegetal organism cease, making it susceptible to parasitic invasion –
hence the necessity for the use of pesticides. Biological agriculture, on the
other hand, considers which elements should be brought to the plant to be
transformed into those elements found in the final stage of metabolism. The
agriculture of the 19th and 20th centuries is dying, whereas biological
agriculture is now taking hold.
May the reader take heed against the so-called
"verifications" made by past professionals of agronomy. These
findings are often illusory.* Analyses have shown discrepancies of 30 to 300%
in all that has been published – even by official document.
[*See French Academy of Agriculture Bulletin, 1970.]
It is quite sad that such data could possibly have been published,
for they manifest clearly the incompetence of many laboratories. The figures
are too exaggeratedly different and contradictory to be taken seriously. I
shall not expatiate further upon this unfortunate example which betrays the low
level at which some research workers are operating. However, I shall return to
these errors later.
Let us take care of the work ahead of us and be unperturbed
by skepticism. We are meeting with opposition that is increasingly animated;
yet one only reacts against that which has become a threat. As a friend wrote
me, "The vigor which you are meeting is beneficial. The weight and worth
of the antithesis reveals the certainty of the thesis."
* * * * *
Agronomists have always recognized that virgin soil, never
before cultivated, will provide a good harvest without the aid of manure or
fertilizer. Little by little, however, deficiencies arise because some specific
elements are found missing after the harvest.
These agronomists have noticed furthermore that allowing the
earth to rest (lie fallow) for a few years restores its lost elements. They
solved the problem by establishing a rotation cycle in which a fallow period is
planned. Leguminous plants such as clover and lucern-grass are sown and they
"fix" the nitrogen, thus shortening the necessary length of the
fallow period.
Agronomists explain this phenomenon by saying that the missing
elements are brought by dust, by animals, by migrations through the soil, etc.
These are simplistic explanations, neither followed up nor confirmed by any
sort of experiment. To say that these "ghost" elements come from a
progressive solubleness of the insoluble composites of the soil is another
simplistic assertion. In order to discard objections of this sort I have
presented data concerning the soluble and insoluble. The traditional notion of
solubility is far too arbitrary: an insoluble composite may be dissolved by
the secretion of radicles, by microorganisms, etc. It is a mistake to separate
the soluble from the insoluble in the laboratory. The acid arbitrarily chosen
to dissolve the mineral compounds of the soil has nothing in common with the
multiple and specific acid secretions of the radicles, nor does it have
anything in common with the microorganisms of the soil which dissolve these
elements insoluble in water but soluble in acids.
Around 1600 a Flemish chemist, Jean-Baptiste Helmont,
planted a tree in a pot containing 200 lbs. of earth. After five years the tree
had gained 164 pounds. The earth had also gained weight, but only two ounces.
Helmont had not supplied any mineral elements, taking care only to water the
plant. He hoped to prove that water had become solid matter.
This summary experiment teaches us little, but it is a case
where the origin of matter stimulated research.
In the mid 19th century in Nantes, Grandeau demonstrated
that a field, which rests a few years, tends to regain its balance. If the soil
is too acidic or too basic, it eventually becomes neutral. Garola confirmed
this observation at the end of the 19th century. In Germany Rudolph Steiner
started experiments of this nature and in 1925 founded a "school of
balanced agriculture" in Switzerland. The Swiss Pfeiffer took over. His
books were published in French. The one most relevant to our concern is Fécondité
de la Terre,* and here are a few examples of his observations:
[*Edit. Les Triades, Paris 1949 (réédit. 1966).]
Lawn and daisies
In order to produce a beautiful English lawn it is necessary
to have earth-containing limestone. Whenever limestone is missing, daisies
spring up in the lawn. For a gardener that is the signal to correct the soil.
The less limestone there is, the more daisies. Pfeiffer analyzed the ashes of
the daisies and verified that they were rich in lime. Since the daisies grew
precisely when there was no more lime in the soil, Pfeiffer asked himself,
"Where did this lime come from?" He could not find the answer.
One could not say, of course, that lime came from migration,
for in that case the grass itself would have had some too.
For Pfeiffer this provided an example of the soil's natural
tendency to find balance. When lime is missing, plants poor in silica grow and
their ashes are rich in lime. Hence when stems and leaves fall in autumn, the
soil gains the missing lime. These examples of interactions are frequent in
nature. However, a mystery persisted: what was the source of the lime in the daisies?
Various observations
Pfeiffer's work raises other questions. Buckwheat has a
marked affinity for sand; silica is very rich in calcium. Let us examine why.
Wheat likes soil relatively rich in lime. The incineration
of its straw gave, for a specific field, a weight of ashes that was 6% of the
original dry weight of the straw. In these ashes there was 5.8%, lime and
67.5%; silica. On the other hand, if clover mixed with the same wheat is sown
in the same field; the clover – which grows better in siliceous soil – gives
35.2% lime and 2.4% silica in its ashes.
The amount of silicon and calcium in most plants is independent
of the amount present in the soil. The plant's composition is constant,
depending on the species (at least roughly constant, for the quality may vary
with the soil). Cultivated digitalis (fox glove) may not contain digitalis;
parsley may not have vitamin D, etc.
The oak, a tree indigenous to granitic or slaty regions
(i.e. soil rich in silica) where limestone is sometimes completely absent, may
contain limestone, largely in its wood and bark. (Up to 60% lime has been found
in its ashes.)
The engineer Simoneton reproduced the following already
known experiment:*
[*1 Research by Lakhovsky, "La Matière" and
"Le secret de la vie."]
Slips of geranium grow quite well in a sand of pure silica;
they are watered with rainwater or distilled water, with no organic element
added and with no other mineral but silica. An analysis of these plants shows,
however, that they produce lime in addition to other elements. Fresh sand
containing bacteria is used, not sterilized sand. This sand, called
"pure," contains, besides silica, traces of seven elements
(representing 0.17 %) of which 0.15% are iron oxides, titanium, calcium and
aluminum.
To conclude this part of the chapter let us cite one of the
numerous experiments made on the English experimental farm of Rothamsted by
Lauwes and Gilbert, two great scientists of the second half of the 19th
century.
A commercial garden produced a culture exclusively of clover
for 17 years. It was mowed two or three times a year and was sown with seed
every four years without the use of fertilizer. Regarding this experiment
Lauwes and Gilbert write, "A singular fact was verified ... this piece of
land, devoid of fertilizer, gave cuttings so abundant that if one were to add
what had been taken away over 17 years, one would arrive at these figures:
2,636 kilos of lime, approximately 1,255 kilos of magnesia, over 2,150 kilos of
potash, approximately 1,255 kilos of phosphoric acid, and 2,636 kilos of
nitrogen."
Figures from a study* made in France in 1955 indicate that
the plants took 1,500,000 tons of potash per year. 300,000 tons were provided
by manure and 450,000 by potassic fertilizer. Hence the fields were given only
half of what was taken away from them. The soil does not contain such reserves
of potash. How then could 750,000 tons be taken every year?
[*Reinberg, Le Potassium et la Vie, P.D.F., Paris 1955.]
The reader understands better, now, that plants have two
ways of producing potassium: from sodium, by the reaction sodium + oxygen =
potassium, and from calcium, by the reaction calcium – hydrogen =
potassium. I would like to recommend
the research recently conducted in Ohio by J. Benton Jones, an account of which
appeared in Science magazine (4/2/65, p. 94). An experiment of this sort opens
a new channel for research. But here again we find verification of a
phenomenon without any explanation.
The oligo-elements (trace elements) are indispensable. They
enter into the structure of the coenzymes. If there is no metal in the
coenzyme, the enzyme remains ineffective. With these important oligo-elements
molybdenum (Mo) is found. Mr. Jones discovered a deficiency of potassium in
leaves of hybrid corn and at the same time verified that the leaves were too
rich in molybdenum.
Plants
deficient in potassium Normal
plants
Potassium
(%) Molybdenum (parts/million) Potassium Mo (ppm)
0.97 2.0 2.70 0.5
0.56 4.0 2.49 0.9
0.52 2.8 1.73 0.7
One can see that under a certain level of K there is an Mo
decrease.
After that, Mr. Jones made a systematic study by changing
the doses of potassium given to the soil. Here are the results, which I shall give
in kilograms per hectare (1 hectare = 2.471 acres). (See Fig. 12)
Potassium Degree
of Composition of the
given to the soil
deficiency in K leaves
.
(K %) Mo
(ppm)
0 severe 0.57 4
36 light 1.79
1.2
72 null 2.28
0.9
108 null 2.49
0.9
The amount of molybdenum does not change when there is
adequate potassium. The diagram shows the simultaneous variations of
potassium, calcium, magnesium, and molybdenum. Although the author makes no
comment, the reader will rapidly make the comparison. Calcium diminishes, but
potassium increases. Let us note that the calcium and magnesium are almost
parallel. This can be explained: the plant needs calcium to make the magnesium
of its chlorophyll, and the more calcium is given, the more the plant enriches
itself in magnesium (up to a detrimental limit, of course). Conversely, if
there is not enough calcium, there will not be enough magnesium. It should be
noted that even without any potassium being provided, it will be found in the
leaves, but four times less than if it had been given abundantly.
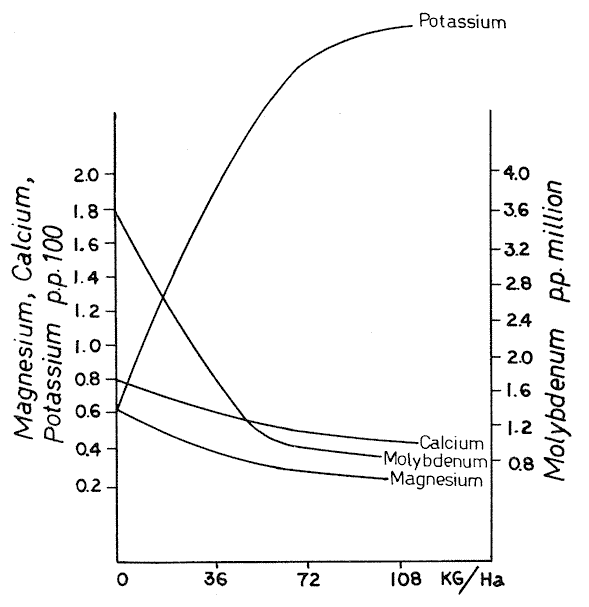
Fig. 12 Variations of
K, Ca, Mg and Mo in corn leaves, depending on the K input in the soil
(according to Benton Jones).
This experiment leads us to a general statement: the origin
of the molybdenum cannot be detected. But an interesting conclusion to which
this experiment brings us is that a plant rich in potassium will be poor in
molybdenum (one of the most important oligo-elements).
Agronomists should then know that an excess of potash, even
if it is supplied by manure, leads to a deficit in molybdenum. It is well known
that a fruit sickness called "bitter pit" in the U.S.A. results from
an overly rich supplement of K in comparison with Ca. Tomatoes have a Ca
deficiency if too much K is present.
One may compensate with Mg which, becoming Ca, will reestablish
K/Ca.
If potassic fertilizers are exhausted some day it would not be
so catastrophic for the agronomists who use them. They may be obtained either
industrially or directly from the soil in at least two ways. Yeasts and
microscopic seaweeds can produce potassium from sodium; other microorganisms
can produce it from calcium.
Already a few companies are producing microorganisms to be
utilized in agriculture. Yeasts of all kinds are made industrially, molds
(penicillin, etc.) as well.
The problems of deficiency in animals and vegetals require
closer study. Cattle breeders and agronomists will be forced to recognize the
phenomenon of biological transmutations, a phenomenon that, although everyone
has already observed and used it, is not understood – thus its application has
been limited. This is the prediction of the leaders of all the associations,
which advise and apply the biological culture in France, Italy, Switzerland,
Germany, England, etc. These leaders are the elite of the agricultural world.
They have observed that chemistry does not explain all of biology, that too
much confidence in chemistry, where biology is concerned, is an error
responsible for much damage.
Excess in any domain is inevitably paid for one day or
another. We are often advised to use 250 kilos per ha (100 kilos per acre) of
potassic fertilizers at 50% K2O,
or 625 kg/ha of sylvinite at 18% K2),
although cereals on the average do not take away more than 40 kilos per ha of K2O per year. Thus ¾ of the K2O is lost. How much land is lost in
America! In the west of Europe the harm is less visible for the time being, for
the common sense of the peasant has helped to postpone the reckoning.
The development of parasites is also a consequence of biological
imbalance. Thus agronomists have had to consult their colleagues who
understand the necessity for developing biological culture.
The mechanism of these biological transmutations teaches us
what to give the soil, according to the following conditions: that the soil be
alive, that it be rich in microorganisms, and that the proliferation of the
latter be possible. If the soil is too much damaged by chemical abuse, one must
reconstitute it. This can take time, especially if there is no humus (an
essential part of living soil).
A simple experiment: the formation of calcium in the
germination of cereals
Here is a simple and convincing way to demonstrate the validity
of biological transmutation. For practicality I have chosen to give a simple
and essential experiment with germinating seeds. (Experiments on man and
animals are very complex.) The experiments involving microorganisms give rise
to problems, which can be handled only with great skill.
Many kinds of seeds have been used and the variation of different
elements has been studied. Nevertheless, for the sake of illustration it would
be better to limit ourselves to an experiment in which the variations are quite
great. Here is our example: let cereals germinate in a medium without calcium
and witness the increase of calcium after germination.
The author conducted this experiment using wheat and oat seeds
from a biological culture. One hundred hand-selected homogeneous seeds were
incinerated in order to determine their calcium weight. (The figure obtained
was compared to the average weight found in different seeds, from dozens of
analyses of thousands of seeds. This was done in order to insure that no grave
error had been committed during the manipulation or analysis of this 100 seed
lot.)
One hundred identical seeds were germinated in vats, on porous
ashless paper saturated with a fertilizing solution of salts dissolved in
water. The solution was free of calcium. After six weeks the vegetation was
arrested by placing the young plants in an electric oven. The plants were then
incinerated and analyzed in the same manner as that used with the first lot of
seeds.
More than twenty such experiments were performed, essentially
on oats, by changing the moment when the sprouting was begun, extending or
shortening the time of culturation, or using different varieties of seeds. Here
are the results obtained. The values are given in milligrams per unit (for
every seed or for every plant). For lots of one hundred seeds, these numbers
must obviously be multiplied by 100. One may operate on lots involving many
hundreds of seeds.
Wheat Oats Oats
"Roux clair" "Noire du Prieudé" "Panache de Roye"
Weight 32.98 37.96 24.48
Calcium (in one seed) 0.0386 0.0372 0.0235
Calcium (in one plant) 0.129 0.155 0.106
There is 3.34 times more calcium in the wheat; 4.16 and 4.51
times more calcium in the oats.
Augmentation of this kind cannot be the result of errors in
computation of results. Nevertheless, the chemical analysis was confirmed by a
modern and specific physical method of great sensitivity: analysis by
spectrophotometry of atomic absorption. The converging results were
communicated to the Head of Agriculture of France on the 1st of December 1971,
by the chemist-engineer Z. E. Zündel.
Commentary
These numerical values ask for some remarks. In biology one
should never generalize. These experiments have shown us that calcium increases
in great proportions in some plants. Every plant reacts differently (or,
rather, every species of plant). That is why the increase is greater for oats,
whereas in some plants – ryegrass, for example – the calcium does not
increase. Hence we learn a lesson: plants able to make their own calcium can
grow very well in clayey and siliceous soil, whereas others cannot make their
own calcium and must be given it. (The variations of Mg and K in oats were also
studied.)
Other observations of ours have proved interesting to many
scientists. For instance, the effect of the moon is very important for the
formation of calcium. In the previously mentioned experiment, the
germination operation was begun at the new moon and terminated at the second
full moon, which followed in approximately six weeks.
I can give only an outline of this relatively simple
research on the germination of seeds. However, I remain at the disposal of all
those who can afford to reproduce such an experiment, to whom I can give all
the useful details. If they do not have the material means for the analysis,
they may entrust the analysis of their seeds and plants to a laboratory
equipped for such research.
The importance of such an experiment in terms of its application
is in knowing whether the calcium increase occurs during the germination only,
or whether the whole plant becomes richer in calcium (to the detriment of
another element which is transformed into calcium – here, potassium). In the
latter case the soil's composition would be modified by the stubble remaining
in place after harvest. There is here a very interesting study to be made by a
laboratory of biological agriculture.
I have reported the qualifying observations made by Pfeiffer
on different plants (e.g. the daisies in the grass), which enrich the soil with
limestone. Another example, very well known by horticulturists, is the case of
azaleas. A culture of these plants was made in a soil of heath, an acid soil
devoid of limestone. The soil analysis showed that it became too rich in
limestone and the cultivation could be continued only by removing a layer of
soil and replacing it with the soil of heath, or by cultivating plants, which
cannot make calcium. This example is another illustration revealing a
substitution of calcium with another simple body, which would have to be
determined in the case of every cultivation.
The whole secret of biological culture is yet to be
discovered with the study of substitution. This phenomenon cannot be studied by
reason alone; it must include concrete experimentation.
The present form of agriculture, to which our biological
agriculture is opposed, leads to the ruin of soil and heath and will
eventually bring about the death of humanity. Already man is poisoned by all
sorts of pesticides or by mineral excess. Phosphates, while favoring the
proliferation of some plants, which invade the waters, simultaneously deprive
the water of oxygen and, little by little, make all life – animal and vegetal –
impossible.
Let us then heed the advice of the specialists of
agro-biology in order to insure the good health of the vegetal kingdom and of
man.
XXI NUTRITION
It is universally known that one of the chief concerns of
the times is the problem of nutrition. Vitamin pills have great success among
those who are not sure that they are getting the proper diet. The source of
protein is a mystery quickly solved by the nutritionist who gives his impatient
patient as much protein as he fears he needs.
Theories about nutrition are based upon the old academic
chemistry, which declares that nothing is lost, and nothing created;
everything is transformed. Therefore, to be safe, extras of everything are
given – plus always a little something in case of a leakage. Thus a chemical
accountancy is forced upon modern man whose mind is centered on the
capitalization of matter. Health is synonymous with capital. Proteins, minerals
and vitamins are caused to be retained internally in any way possible. If the
iron's desire is to leave the body, one employs a fixative so that it will
remain there long enough to provide energy for meeting the day's activities.
In such a way chemistry has sold its soul to the merchants, the muses of the
research workers; these workers are told what to produce in accord with the
needs of the times.
Medical doctors often do not know the contents of the drugs
they prescribe. Merchants provide them with brief pamphlets, which are too
seldom read. More time is spent in the politics of big business than in curing
the patient.
It is hoped that biological transmutation, which is intended
for everyone's understanding, will awaken man from his lethargy. There is no
need to surrender oneself to strangers who aim to manipulate consumption during
the next decade. This new science with its clarity of exposition, its
thousands of proofs, and its chief witness – the order of the world – will
guide man in such a way that he will be able to choose his food himself,
according to his own needs.
* * * * *
The findings concerning calcification alone should suffice
to convince anyone concerned with proper nourishment, about the significance
of biological transmutation. It does not help in any way to take into account
the mineral calcium found in the food, for our organism rejects the majority of
this calcium and fixes the rest of it but imperfectly. (When it is hot,
especially, one rejects more of this element than one ingests.)
The phenomenon of transmutation, unobserved by dietitians,
proves the caloric balance sheets based solely on the chemical reactions of
carbon oxidation to be inadequate. The research done with the calorimeter will
not prove satisfactory since some elements, due to enzymes and various
physiological conditions, can be transmuted with absorption or emission of
energy. This is enough to show how incompatible are the complex determinations
of the energetic balance sheets, setting forth the simplistic view accepted to
this day by almost everyone.
We have seen the impossibility of relying completely on
chemistry. Several medical doctors understand that a diet rich in calcium does
not necessarily strengthen the bones. Decalcification is sometimes caused by a
deficiency of the enzyme, which transmutes sodium into magnesium. If, however,
it is due to a deficiency of the enzyme, which transmutes Mg into Ca, it is advisable
to strengthen the bones with potassium and organic silica. When calcium is
being used by plants, this element, with the help of other enzymes, may produce
potassium and magnesium (as do the bacteria of saltpeter).
Decalcification may then occur when salt-free diets are prescribed,
especially chloride-free diets.
Silica seems to be a superior element for the strengthening
of the bones. One must still refrain from excessive intake, as with any kind of
food, but the tolerance is great. That is why it can possibly be used without
medical control. The excess is eliminated by the urine, thus horsetail is a
diuretic for many people.
The action of horsetail is rapid: nails which break
easily, an early sign of decalcification, become normal within two weeks with a
horsetail extract; horsetail decoctions take a while longer.
Spectacular results have been obtained with fractured bones.
An experiment was made in a specialized nutritional laboratory where young rats
were subjected to a controlled diet.*
[*Transmtttation à Faible Energie (2nd edit.), Maloine
Pub., 1972, p. 100.]
Fig. 13 Action of vegetal silica in recalcification.
Broken femurs of rats – 10th day.
Photos 2 and 3: controls receiving a diet normal in calcium.
(One can see that the repair has lust begun.) Photos 4 and 5: rats receiving a
supplement of vegetal silica. (The soldering is neat and the callus is
forming.)
These rats were divided into two lots of three each. One of
the lots received a normal diet, which included a fairly generous amount of
calcium. The other lot had an extract of horsetail added to its food. X-rays of
all the rats were taken ten days after their bones were broken. It was clear
that the ingestion of vegetal silica had already healed the bones. An X-ray
taken on the 17th day showed that the cure was complete, whereas with calcium
alone, X-rays taken on the 17th day showed that the bones were not yet healed.
Fig. 14 Action of vegetal silica in
recalcification. Broken femurs of rats – condition on 17th day.
Photos 2 and 3: slow progress in controls (diet with normal
amount of Ca). Very little change in 7 days.
Photos 4 and 5: rats receiving a supplement of vegetal
silica. Almost complete soldering. The callus shows from its opacity that the
limestone density is greater than in the remaining part of the bone, which is
transparent (4).

– Horsetail –
On the other hand, Professor Delbet demonstrated many times
to the Academy of Medicine that it is beneficial to increase the usual
allowances of magnesium. The cases of decalcification, much more frequent
nowadays, are due in part to our "industrial" modem diet, which is
deficient in magnesium. White bread and white salt are preferred for business
and aesthetic reasons, to the detriment of our health.
It would be beneficial if a systematic study were made,
under medical control, in nurseries, kindergartens and high schools. There is
much to be learned from the conclusions that would be made concerning the food
that is provided there.
Statistics taken from January 1957 to November 1963 in Copenhagen
showed that 80 children, from three to four months old, died suddenly.
Although they had been given milk, their deaths were caused by a lack of
calcium. Accidents of the same type were observed in a hospital in Paris. A
post-mortem study showed that these sudden deaths, occurring in the cradle
without warning, were caused by a spasm of the glottis, which turned
upside-down and obstructed the trachea.
Many hospital dietitians and even pediatricians do not yet
know that these spasms, which are caused by a lack of calcium in the
contractile cells of the blood, cannot be fought with calcium. Only magnesium
can cure these spasms; the same applies to rickets. It seems that these
unfortunate children had a deficiency of an enzymatic order; therefore, they
had difficulty in transmuting sodium into magnesium. They should have been
given more magnesium directly. Of course, one should be careful to give a
supplement of magnesium to patients subjected to salt free diets, to avoid
decalcification.
Cereals and whole flours are richer in magnesium than meat
products. We do not advise taking magnesium in the form of sulfate, this salt
being purgative and irritating to the mucous membrane of the intestines.
When animals travel by truck or rail it is advisable to
triple their intake of magnesium a few weeks before the trip, in order to
strengthen their skeletons. This will permit them to travel without the risk of
a fracture, especially the pig and calf that, due to modern methods, have
"grown too fast."
More could be written about dietetics in the light of the biological
transmutations. It is expedient to expose the problem of decalcification since
that is the most classical among the problems of nutrition. The reader will
have to go back to previous chapters if he wants to understand more about the
subject, succinctly treated here. In order to understand the problem of
decalcification he will have to study closely all of the chapters in which
calcium is involved. Once again, this book is intended only as a catalyst; its
goal is to arouse in the reader – and hopefully in the minds of scientists –
enough curiosity so that they will pursue the subject on their own.
I shall end this chapter with a story, which illustrates in
the most dramatic and clear manner the problem of nutrition.
A doctor advised a pregnant woman to take iron when he discovered
that she was deficient in this element. But because iron cannot be fixed in the
body he also advised her to take with the iron something to fix it in the
intestines. The woman, who did not believe in taking too many pills, was
somewhat shaken by the "news" that she was lacking iron. She went
home and told her husband, who was well acquainted with the phenomenon of biological
transmutation. The husband reassured her that everything was all right, but
that she would simply have to take more cereals and chew them more thoroughly
in order to assimilate the manganese well and activate her metabolism. The
husband then advised her to make her next appointment with the doctor in the
afternoon instead of in the morning. A month later she went to the doctor, who
declared that she had done well to follow his advice for there was plenty of
iron now!
What happened was this: cereals (especially whole wheat,
brown rice, etc.) are rich in manganese. But we know that manganese changes
into iron (Fe56 – H1 =>
Mn55). The pregnant woman had
a slow metabolism, so her husband advised her to see the doctor in the
afternoon; by that time the manganese had changed into iron. A woman with a more
active metabolism has plenty of iron within a few hours after breakfast.
Iron content varies with every individual and must not be administered
mechanically. The biological transmutations promote the serenity of the
physiological man as compared to the paranoia of the world of the
"quick" pill.
XXII MEDICINE
A time will come when the biological transmutations will be
fully instituted and practiced in medicine. The health that modern man has is
temporary, lasting just for the time it takes for the last pill to wear off.
When a symptom of a sickness begins to appear one shuts it in mechanically with
whatever new pill is on the market. If one unit is not enough, one takes two,
etc. – for months, or even for life. Rather than seeking quality, one turns to
quantity. Quantity overwhelms the sickness and baffles it for a while, but like
a balloon pressed against the water, the sickness springs out stronger than
ever in another place. In medicine, as it is practiced by many doctors, there
is no intelligence involved. Everything is arranged so that nothing will be
lost. When something disappears, everyone is worried and hurries to replace it
immediately – and, to insure the success of this mechanical operation, a
supplement is administered. From a comprehensive physiological understanding
man has shifted to the purely mechanical. The body is but a factory without a
foreman where all the machines run wild. A man is hired to put things together.
But the task is so difficult that he gives up the job and another is hired.
The "replacement" of an element is a primitive
approach, born out of defects in understanding. This mechanical attitude makes
man a frightened being who is out of place in this world. This pattern of
thinking and practice impedes progress and, worse, leads man to the loss of his
mental faculties. With the proper understanding he could evolve and become the
man he is meant to be, not a compulsively busy one, wasting his time patching
up his sorrows and pains.
The phenomenon of biological transmutations brings man back
to life and reality. By studying and making use of them man will recover the
health he lost a long time ago. Health means not only to be able to do one's
work with a certain required strength, but also to be a clear thinker who can
spend time learning about the wonders of this world. To be healthy is to be
able to grow and perceive; it is also the ability to learn from others with
humility. An artificially induced physical health is temporary. True and
complete health must encompass the mind as well as the body; it implies
happiness and understanding. The application of the biological transmutations
can be a guide for the safe conduct of man.
Calcium is not always the answer
The problems of decalcification and the strengthening of the
bones must be investigated all over again. We have seen how fractured bones can
be healed rapidly by means of organic silica (see chapter entitled
"Production of Calcium From Silicon"). We have learned how horsetail,
which is quite poor in calcium, can help heal fractured bones. Spring
horsetail, as compared to summer horsetail, is rich in organic silica – not in
mineral silica, which is, on the contrary, decalcifying.
Fresh green vegetables (young plants), radishes, etc.,
contain a large amount of silica. We now know it is due to the ingestion of
fresh grass that milk-cows can excrete more calcium than they ingest, without
decalcifying. The pregnant woman who breastfeeds her baby may correct her diet
by adding a small amount of horsetail to her food in order to avoid
decalcification. This is now recognized without dispute and is being applied
commercially in France.
Many articles include names of authors* who have referred to
my work, citing spectacular results obtained in the attempt to strengthen the
bones with organic silica. The chief surgeon of a hospital asked for my
assistance when he found himself confronted by a delicate case: a young man
with bones broken very badly in an accident. The classical treatment of Vitamin
D plus a phospho-calcic salt failed to bring about any improvement. However,
the administration of organic silica healed the bones rapidly. I could cite
various other examples.
[*R. Haegel, "Calcifications et Transmutations
Biologiques," La Nouvelle Hygiène, Paris, Sept. 1966.]
At that time Professor Delbet had already arrived at the
understanding that it does not help much to ingest calcic phosphates. He
wrote, "It is questionable whether calcium phosphate is formed in the
bones," and, "we do not know how the calcic phosphates come to the
skeleton," for calcium has never been found approaching the bone.
In 1962 a Canadian author, H. Selye, wrote a whole book
about what he called "calciphylaxis," which he defines as "a
diffuse hypersensitivity" in which "the tissues react ... because of
an intense and local calcification" – a purely dialectical explanation.
Unfortunately, he concludes, "The nature of the local mechanism of
calcification is one of the most important problems of biochemistry which has
not been resolved." He had no knowledge of my work.
The question of calcification commands us to revise all our
notions about dietetics, for it is useless to take into account the mineral
limestone found in food. Our organism rejects most of this limestone and fixes
the rest but poorly. In general, one rejects ingested calcium when it is hot.
The mineral limestone comes in part from magnesium, when the latter is produced
in excess.
Charnot's research*
In the autopsy of a tuberculous patient, Charnot noticed a
strengthening of the bones and a simultaneous anomaly in the percentage of
magnesium, accompanied by a lack of silicon in the bones.
[*"Le Silicium dans l'Osteomalacie le Dannous et la
Turbulculose," C.R. Acad. Med., Paris 1947.]
Magnesium and silicon are two of man's main sources of limestone.
The verification of this fact served as the starting point for systematic
research on tuberculous guinea pigs, establishing that tuberculosis is always
accompanied by decalcification. Silicon disappears before calcium; that is an
early sign of decalcification. But when organic silica is given, a rapid
calcification of the caverns occurs.
When silica disappears, calcium leaves the bone. If organic
silica is administered, the calcium returns. Whatever interpretations one may
conceive, one fact is irrefutable: the two elements are linked.
Charnot also remarked that there is a link between potassium
variation and decalcification. (See chapter entitled "Potassium
Calcium.") This fact inspired him to utilize the actions of silicon and
potassium in the treatment of rheumatism.
To a patient whose rheumatism was so bad that it deformed
her fingers and joints, Charnot gave bicarbonate of potassium with an extract
of organic silica. An X-ray was taken before the treatment. After 6 days of
treatment another X-ray was taken. It revealed a complete cure.
To another patient, suffering from a decalcification of the
knees, he again gave organic silica, this time extracted from plants. The
silica was given with bicarbonate of potassium. After a month of daily
treatment the knee was healed.
To a woman who was badly decalcified he gave, for several
consecutive days, an amount of calcium superior to the amount she was
excreting; nevertheless, she excreted more silica than she ingested.
Though one can learn from this that potassium and silicon
bring calcium to the bone, thus curing rheumatism without having to depend on
the ingestion of calcium, he should be careful not to generalize. Excessively
heavy doses of silica – especially the mineral ones – cause decalcification.
Charnot's experiment is cited here as an example for the reader of how
biological transmutation occurs in man's body.
The impracticability of an absolute reliance on chemistry
has become clear to several doctors. For example, a diet rich in calcium does
not necessarily strengthen the bones properly. This is the understanding of
those concerned about promoting efficient diets. One of them, Dr. Plisnier
(Belgium), made various observations, which make sense in the light of
biological transmutation. In his book "Save Your Health" he states a
few observations, which I can cite but incompletely:
a) A delayed dental growth in children
receiving a diet normal in calcium (according to the old, classical dietetics,
which prescribes fruits, vegetables, milk, cheese, and meat) was corrected in a
matter of a few weeks. The children were given instead a regimen excluding milk
and cheese (which are considered great sources of assimilable calcium).
b) This same diet, poor in calcium,
allowed the rapid repair of a fracture. The patient was a woman in her sixties
who had broken her femur, which, in spite of two operations and a diet rich in
calcium, could not be healed.
In medical reviews, authors are universally recognizing the
contradictions, which present themselves – concerning sodium, potassium,
magnesium, or, most of the time, calcium.
Let us not accept blindly the dictums taught us. We have
been told that the practice of substitution is essential, that if calcium
appears it is because it has been displaced – by magnesium, for example. This
is a position taken for the sake of conformity, to satisfy the
"non-creation" dogma. Those who affirm the substitution theory have
never weighed the magnesium and calcium before and after the experiment.
Those who believe in the substitution theory say that the
total Mg + Ca is constant. This is an unfounded assertion, for the relative
constance they have observed results from the fact that Mg decreases and Ca
increases. The sum of Mg + K + Ca is constant. But as far as some scientists
are concerned, the element lost from an organ has gone to some other place!
I remind the reader of the experiments made in the Sahara
Desert where for six months petroleum workers excreted an average of 320 mg
more calcium every day than what they ingested, and this without
decalcification!
All that has been written on the metabolism of calcium,
which does not take into account the biological, transmutations must be studied
all over again, as there are important facts medicine must begin to use.
The thyroid and the metabolism of calcium
In the chapter entitled "Sodium and Potassium" I
indicated that the transmutations of the latter seem to depend on the
aldosterone. But in the chapter devoted to magnesium, I made no allusion to the
endocrinal secretions on which the magnesium-calcium link may depend. In order
to orient research, which would help determine the enzyme capable of
accomplishing the transmutations, it seems useful to cite a few findings:
The role of the thyroid in the regulation of mineral
metabolism has been studied in vertebrates and especially in fish, by M. Fontaine
(1954).*
[*Archives Soc. Physiologie, 7, C.55 to C.78 (Paris).]
The lowering of the water salinity of the outside medium
stimulates the thyroidian formation of the fish, says M. Olivereau (1948),
whereas the opposite, the elevation of the salt rate in the inside medium,
makes the thyroid decrease its activity.*
[*C.R. Soc. Biologie, p. 124, 176 (Paris).]
Nigrelli (1952) believes that it is the variation of the
Ca/Mg ratio in the water which influences the thyroidian activity of the
fish.* This is also recognized by
Professors Berg and Rasquin; Olivereau admits it also (1955). Etienne (1958)
studied the actions of the K, Mg, and Ca ions on the thyroidian function of
trouts.** There are many others, such as Koch (1942), who studied the influence
of the thyroidian hormone on the osmotic regulation of the fish.***
[*Zoologica, 37, 185-189 (Paris).]
[**C.R. Soc. Biologie, 152, No.2, p. 308-312 (Paris).]
[***Ann. Soc. Roy. Zool. Beige, 73, p. 164-172.]
Hence many individual discoveries have shown that the enzyme,
which regulates the relation between Mg and Ca, seems to be secreted by the
thyroid. The stimulating action would depend on Mg, which leads one to believe
that Mg provokes an increased thyroidian activity.
This dependency of the Ca/Mg ratio on the activity of the
thyroid is extremely variable according to the species of fish, all of which do
not react in the same manner to a concentration of the Na, K, Mg, and Ca ions
in the water. It seems that this dependency is apparent – in varying degrees –
in all vertebrae; in the case of man, Leriche has verified links between calcic
excess and hyperpara-thyroidism.
A fault in calcium metabolism thus seems to be linked to a
modification of the thyroidian and para-thyroidian activity.
All endogenous formations of calcium seem to be connected
with the thyroid and parathyroid. The action of K has also been studied: K
increases thyroidian activity, as does Mg; Ca does not.
But calcium may also come from silica (and there could be a
simultaneous action of Mg, K and Si, producing Ca) so that one must find a link
between organic silica and the activity of the thyroid.
It has been established that people with tuberculosis suffer
a decalcification. Dr. A. Charnot proceeded with an analysis of 29 organs or
parts from both a healthy guinea pig and a tuberculous one so he could weigh
the silica. Here are some results, given in grams for 100 grams of fresh
organs:
Organ Healthy
Guinea Pig Tuberculous
Guinea Pig
Lungs 0.0059 0.0058
Liver 0.0053 0.0068
Kidneys 0.0046 0.0055
Brain 0.0093 0.0104
Heart 0.0072 0.0073
Pancreas 0.0061 0.0044
Thyroid 0.0234 0.0533
Surrenal 0.0418 0.0390
Blood 0.0452 0.0554
Cerebellum 0.0663 0.0686
Teeth 0.0505 0.0463
Bone 0.0491 0.0412
Skin 0.0657 0.0573
Hair 0.0899 0.0530
The silica rate is the same in the spleen as in the
pancreas. Young guinea pigs have a thymus rich in silica.
One can see that in the teeth and bones the silica rate is
high in the healthy guinea pigs but low in the tuberculous ones. This reduction
of silicon precedes the reduction of calcium, as if it were the lack of silica,
which caused the lack of calcium. This is interesting because falling hair and
nails is the early sign of decalcification.
One can see that the thyroid is relatively much richer in
silica than the other organs. Here the tuberculous subject has a silica rate
twice as high as that of the healthy subject.
This relative abundance of silica suggests that the thyroid
plays an important role in the metabolism of calcium. I do not think that this
observation has been made before. That is why it will be fruitful to compare
all the research made concerning the role of the thyroid in the metabolism of
Mg, K, Si, and Ca.
There are some other cases where the action of the thyroid
could be associated with magnesium. I have already stated the variations of Mg
in cancer, as cited by Delbet. If the thyroid is removed when the cancerous
tumor is operated on, the reappearance of cancerous tissue is much more
pronounced.
Would this not come from the fact that the regulation of the
Mg metabolism together with that of K and Si is perturbed? Wouldn't this be an
ideal ground for the cancerous proliferation?
Heart failure
Groups of M.D.'s (some of them professors at the Faculty of
Medicine) wishing to learn more about the basic problems of their discipline
asked me to teach them the mechanism which had so far remained unexplained by
biochemistry: the potassium increase in the blood despite a diet deficient in potassium.
They had read my first books where I explained how the endogenous production of
potassium was possible. This knowledge had opened for them new horizons in the
explanation of the mechanism producing heart failure. (Death occurs when there
is an exaggerated increase of potassium in the blood serum.)
It is known that the body cell (the nervous cell is no exception)
is rich in potassium, although it lives in the midst of a nourishing liquid
rich in sodium.
The potassium can go from the inside to the outside of the
cell wall (called cellular body) and back again. In some cases the same is true
with sodium – a normal balance is achieved by a much higher potassium content
inside the cell. Because of this, there is outside the cell an intermediate potential
between K and Na, leaning toward K. In the serum there is a potential leaning
toward that of Na.
Let us recall that compared to that of the hydrogen
electrode, the potential of sodium is negative: -2.175 volts. The potassium
potential is even more negative: – 2.924 volts; thus K behaves more negatively
than Na which has 0.209 volts. One can imagine a battery where the positive
pole is Na and the negative pole is K. (The reader will recall that the
differences of potential to which the nervous fibers are sensitive consist only
of some tens of millivolts. There is a degree of rest and a degree of
excitation. Thus great mixings of K and Na are possible, since in a pure state
there would be 209 millivolts difference.)
However, if one ends up with a ratio such that the
difference between the potentials on each side of the nervous cell wall is too
small, excitation is not possible. The nerves are blocked and heart failure
results.
In the case of heart failure the potassium increase in the plasma
can rise so high that the difference of potential from that of the inside of
the nervous cell becomes insufficient and there is a blocking of the nervous
influx. This, of course, is a simplified figuration, for the biological reality
is always much more complex.
(For a more proper explanation see ... http://www.hbci.com/~wenonah/riddick/
)
In order to prevent a dangerous increase of K in the blood,
some doctors thought to maintain a severe regimen including a minimum of
potassium. In spite of a significant deficiency of K in the food, the potassium
level continued to rise, causing an eventual accident.
Confronted with such a failure, others thought that the
dangerous K increase in the blood called for removal of this serum too rich in
K and the injection of an artificial physiological serum at 9% of NaCl,
containing no K. Unfortunately, this direct injection of sodium into the blood
circuit provoked an immediate death.
There is, in the logic of the biochemist, another reason for
believing that sodium should be given. When the potassium increased, there
was an intense, unexplained "leakage" of sodium. The subsequent
reasoning was thus: since the sodium goes away and there is not much of it left,
we must give it to the organism! The
nuclido-biological reactions, which I had observed, helped to explain what was
happening. A hormonal disorder (of the surrenal) provokes the continual
synthesis of the aldosterone, thus transmuting the blood's sodium into
potassium.
Hence, in this case the potassium increase in the blood has
nothing to do with the oral ingestion of potassium, because it is the sodium of
the blood plasma, which becomes potassium. It is only the sodium ingestion that
one should change, and it is understood that by an injection of artificial
serum – water salted with sodium chloride – the blood receives a fresh and
abundant afflux, which transmutes immediately into potassium and causes death.
A salt-free diet is traditionally imposed, but for a lasting cure one should
act on the surrenal.
Addison's Disease
In December 1963 a chief endocrinologist of one of the
largest hospitals in Paris asked me to give some doctors an expose on
potassium. This professor wanted to show his assistants, interns, students,
pharmacists, laboratory chiefs, etc. the formation process of potassium and the
disappearance of sodium, since the sodium "leakage" and the potassium
risings remained unexplained. A typical example is Addison's Disease, where the
percentage of sodium in the blood plasma decreases while the percentage of
potassium increases.
It had been established in 1927 by Bauman and Kurland that
the potassium increase in the blood serum was caused by a malfunctioning of
the cortico-surrenal.
In 1931 Hastings and Compere, in a surrenalectomy on a dog,
verified that on the second day the increase of K in the plasma can exceed the
normal balance by 50%, causing death to occur a few days later (at which time
there is six times more K than normal).
The potassium also increases in the cell. Harrison and
Darrow in 1938, Buell and Turner in 1941, verify this in the rat.
This observation invalidates the arguments of people who say
that the increase of K in the blood serum is caused by a "leakage" of
K from the cell. The researchers previously cited provided proof that it is the
total potassium, which increases; it is not a case of the simple removal of an
ion.
In Addison's Disease the symptoms are aggravated by an excessive
supplement of potassium in the diet. The disastrous role of potassium with
regard to cortical malfunctioning has been confirmed on surrenalectomized
animals by Allen, Nilson, Kendall, Cleghorn and Mc Vicar.
The plasmatic sodium, which falls in cases of cortical
malfunctioning, rises under the effects of D.C.A. or cortisone. The plasmatic
potassium, which rises in cases of cortical malfunctioning, is decreased by
D.C.A. or cortisone. The opposite effect, which these have on Na and K, was
established in 1948 by Green, in 1939 by Kuhlman, and in 1940 by Tookes. D.C.A.
can act so intensely that it can cause a loss of the K stored in the cell.
Darrow demonstrated that under the influence of D.C.A. a
deficit of intracellular K causes an alkalosis. This alkalosis (increase of Ca),
linked to K, has been confirmed by specialists of endocrinology.
Thorn, in 1941, demonstrated a different effect caused by
cortisone on Na and K in Addison's Disease. An excess of cortisone (or D.C.A.)
can lead to such a loss of K that decalcification results. It was never
understood why the lack of K caused the lack of Ca!
Rheumatism treatments with cortisone (200 mg/day) modify the
Na/K equilibrium. Long treatments can lead to fatal troubles by causing the
atrophy of the gland, which secretes these hormones (resulting in a reduction
of the cortex matter).
In this treatment of rheumatism, as in the treatment of Addison's
Disease, the amount of potassium excreted is greater than the amount ingested.
This negative balance sheet is accompanied by a negative calcium balance sheet,
showing that the disappearance of K is accomplished directly by the
transmutation of the latter into Ca.
Cushing's Disease
Here, the opposite is true: a hormonal excess leads to a
sodium increase in the blood.
The alkalosis in this disease is corrected simply by giving
potassium.
Cushing's Disease is caused by excess surrenal activity,
which in turn is often produced by a tumor of the hypophysis, no longer able to
control the Surrenal. The latter then produces an abnormal quantity of
cortisone and other steroid hormones. One knows the effects of excessive
injections of cortisone. Excess tension, a loss of minerals in the skeleton
and a poor endocrinian function are the consequences, and together they may
ultimately cause death.
Cushing's Disease has been considered incurable. It has
always been treated by surgery: by the partial removal of the surrenal and the
removal of the tumor in the hypophysis. It has also been treated with gamma or
X-rays, an attempt which always causes damage to adjoining tissues.
* * * * *
People who undergo cortisone treatment typically excrete
more K than they ingest. For us this fact was one of the leads in researching
the enzyme which provokes the transmutation of Na and K and which seems to be
of the same group.
The secretion of ACTH from the hypophysis under traumas such
as hemorrhages, severe burns, violent physical effort, acute infections, bad
wounds, operational shocks, strong emotions, etc., also causes a large
secretion of K. I believe that in this
case we should study the possibility of giving a physiological serum (Na).
Whoever deals with the excretion of K must also consider the Na consumption,
since Na is needed to replace K. Hence water, salted with NaCl, should be given
as liquid intake to avoid a possible loss of K.
Above all, one should not commit the grave error – which is
still being made – of saying that since the organism is losing K, one should
introduce more of this element. (This is similar to returning to an organism
its wastes!) Actually a large injection of KCl could be dangerous because it
might cause a local and lethal concentration in the cells.
It is by using precise balance sheets that we have
established the reality of the biological transmutations. It can be seen that
this "new" science clarifies much that has remained mysterious thus
far (concerning the metabolism of potassium, for example). This evidence must
surely oblige everyone to return to his studies in biology. Indeed, the facts
have been seen for a long time. It was no disappointment for me to learn that
they had been discovered before me. Quite the contrary – I was thrilled, for
this merely proved me to be correct.
EPILOGUE
When one is confronted with a fact,
which is in opposition with a prevalent theory, one must accept this fact and
abandon the theory, even though the latter, supported by great men, may be
generally subscribed to. – Claude
Bernard –
This book is essentially a document of experiments, which I
hope will be sufficient to establish the undeniable reality of the phenomenon
confronting us. It was necessary to limit the treatment of the subject,
leaving the door open concerning the different applications of these newly
discovered reactions. I hope the examples cited here will not only help the
reader understand the mechanisms but will encourage him to proceed in his own
field with each particular problem.
The preceding pages have demonstrated that the biological
transmutations of the elements do not in any way oppose the laws of chemistry.
Chemistry is the science of the displacement of the electrons situated in the
peripheral layers of the atoms. It is the science of the molecules, not of the
nucleus of the atoms. The phenomenon that I have unveiled exists at the level
of the atom's nucleus. Thus it is a new science, quite different from
chemistry, which is simply the end result of the transmutation phenomenon and
therefore possesses certain limitations.
Einstein never stated that his laws were applicable to
biology. He always insisted on the fact that they were relevant only in his
particular field of research.
Open-minded physicists know well that not all the laws of
physics are applicable to living matter, a fact that made Louis de Broglie
write, "It is premature to reduce the vital process to the quite
insufficiently developed concepts of 19th and even 20th century physics and
chemistry."
Along the same line R. H. Dicke in "The Theoretical
Significance of Experimental Relativity" (New York) said that there is
now emerging a lessening of the original infatuation with the proofs related to
the law of general relativity.
More and more physicists now oppose the rigid attitude of
certain specialists. One of these physicists, H. Prat, a professor at the
Faculty of Science, wrote: "In fact all our physical and biological laws
are more or less based on the implicit acceptance of the notion of identity.
They should all be studied over again and made more flexible."
As a further example the eminent physicist Brillouin wrote,
concerning the healing of tissues, etc.: "That is why so many scholars
think that our actual laws of physics and chemistry cannot explain such
strange phenomena."*
[*Vie, Matière et Observation, A. Michel Pub., Paris 1959.]
In the atomic field everything is brought to the level of
Einstein's theory. This confusing attempt to generalize is not being made
universally, however. Other scientists declare, "The equations of
Einstein remain valid, but it now appears that they might be incomplete."
(The Sciences, July 1964). Also, "A new examination, the complete revision
of our ideas, will lead us to the conclusion that there are accommodations to
be made with relativity."
I do not oppose Einstein. I only oppose his unconditional followers
who did not understand their master and who applied in all fields laws whose
capacity Einstein himself had circumscribed.
But who reads Einstein? Many of the popular books on relativity
are written by professors who misunderstand these laws and who continue to
apply automatically the false calculations made by previous authors.
"It is only for these bodies of reference (Galilian
bodies, i.e. those which make a rectilinear and uniform movement, exempt of rotation)
that the validity of the principle of relativity was admitted. ..." wrote
Einstein.* And, "The physical
interpretation of the space and time Euclidean continuum ... was possible by
virtue of the law of constancy of light velocity ... which the theory of
General Relativity could not maintain. ... " Einstein's position before
his death was that there are several valid theories, each completely distinct
and applicable in its own field. Yet now many passionately support Einstein's
theory while others completely oppose it. Physicists are divided, and I have no
wish to take part in their debate.
[*La Relativité, Payot Pub., Paris 1956.]
What is important is that doctors, biologists and
agronomists are already applying the biological transmutations. Some doctors
apply them empirically without completely understanding them. The same is true
with agronomists who leave a deficient field lying fallow for a few years,
after which period the land regains the elements in which it was deficient.
Also, many bone specialists know that the bone is formed without calcium.
The entire genesis and evolution of our planet needs to be
restudied in light of transmutation, which opens new horizons to geologists and
philosophers, as well as to metaphysicians. The latter can find grounds for
meditation in the fact that the vital phenomenon of life is not chemistry
alone.
This does not mean that the recognition of biological transmutation
is another step towards comprehending Life. On the contrary, I would say that
it is a step toward demonstrating that Life is more complex than some
biochemists would like to believe.
"When one makes a general theory," said Claude
Bernard, "the only thing of which we are certain is that all of these
theories are false, absolutely speaking ... for they should be modified with
the growth of science, so much the more since sciences are less advanced in
their evolution. What distinguishes the scholar is not making discoveries in
which chance plays a great role, but finding the laws which govern the
phenomena."
It is the exaggerated specialization in every field of
science, which condemns the modern scientist, isolating him from other
disciplines. For many, science today is just another job, where the employee
has lost his sense of adventure.
Let us again recall that the transmutations at low energy
are a phenomenon totally different from the one actually studied in nuclear
physics. The transmutations observed here were all of a biological nature. They
lead us to recognize that the natural structure of atoms is different from that
structure resulting from the technique used in the study of nuclear physics.
The nucleus set is formed of sub-sets, which can be separated with a relatively
small amount of energy. More particularly, most of the reactions studied can
occur with the movement of an atom of hydrogen or an atom of oxygen, thus with
± H or ± O.
If this is a general principle, however, it must be said
that the group of protons O can neither be separated from one nucleus nor
joined to another. The same is true of H. What is now called "Kervran's
Effect" is thus limited to certain reactions, which are the same in
biology. An example is the metamorphosis of rocks, under pressure effects and
temperature, which have no basis of comparison with the energies of fusion, or
fission, calculated by classical nuclear physics. Hence one may have an
exchange of one proton in 11Na <=> 12Mg or 13Al <=> 14Si. This leads us to the complementary hypothesis that
the above movements of protons would occur only to introduce or take away a
single proton from one of the sub-sets.
Thus it can be understood why in geophysics one observes the
"Kervran's Effect" kind of transmutations and only these, although
laboratories using high pressure for the synthesis of minerals come across the
same transmutations. Our findings are thus confirmed by a different agent and
both converge in the concept of a nucleus formed of "cleavable
parts."
I hope that the reader who has attentively read this work
will be aided by the knowledge of the phenomenon of biological transmutation.
More books will be translated from French into English in the future. These
books will, of course, include new experiments made by those who are now
applying the biological transmutations in their respective fields of research.
In view of this fact the theoretical aspect of our subject has been neglected
in this work.
The
Art of Healing Ourselves
Using
Hydroponics to Understand the Earth's Life Processes
On the Atomic Level
Tommy's
History of Western Technology
"Health
and Light" by John Ott
The Tortoise Shell "Science of
Health" Newsletter
— Putting an End to Disease on Our Planet —
Tortoise
Shell Life Science Puzzle Box – Front Page
View this page Full Frame















